Thank you for signing up

Compressed gases in pharmaceutical manufacturing: best practices in microbial monitoring
Compressed air and other process gases are used in a lot of different steps during pharmaceutical manufacturing. Some examples are the use of compressed air in direct contact with products to clean, aerate, or move them through the processes or the using process gases in fluid pumps that take products through the production and filling processes. Compressed gases, such as nitrogen or argon, can also be used for blanketing or to spray or coat a product. The risks associated with the use of these gases, depend on the amount and type of product contact and based on this risk assessment, a suitable monitoring plan should be in place.

Meet QbD Ambassador and Clinical Pharmacology & Pharmacometrics project lead Thomas Van Looy
At the QbD Group, we want to take into account the opinion of our employees. Therefore, our Board of Ambassadors was elected to represent the voice of all QbD’ers, within and outside of the group. Thomas Van Looy has been elected as one of our Ambassadors for 2022. Congratulations, Thomas! As an Ambassador, he loves to share his QbD Group experiences. Want to find out more about Thomas’ #QbDream job? Then don’t miss out on the interview below.

Data migration in CSV: definition, purpose, and best practices
Sometimes it is necessary to perform data migration in computer systems, applications, or software. Learn more about the goal, importance and risks here.
New GMP Annex 21 ‘Importation of Medicinal Products’ – Are you ready?
On August 21, 2022, the new GMP Annex 21 will enter into force. This Annex contains the GMP requirements for MIA holders when importing medicinal products from outside the EU/EEA. Learn more here.

Clinical Evaluation Report (CER): a must-have for all medical device classifications
To document the clinical evaluation of your device and its output, a Clinical Evaluation Report or CER has to be compiled. Learn more about this key document, its content, common gaps and hurdles.

How to use electronic signatures in regulated industries?
During the pandemic, electronic signatures gained tremendous importance for companies in the regulated sector. Learn more about electronic signatures, their use and safety here.

Clinical Evaluation Plan (CEP): roadmap of the clinical evaluation strategy
The Clinical Evaluation Plan, or CEP, is a crucial starting point and guide for the clinical evaluation of your medical device. Learn more about this key document and its requirements.

What is decommissioning in CSV?
In CSV, decommissioning is the retirement or controlled shutdown of an IT solution or computer system that is no longer needed. Learn more about this process here.

Clinical evaluation for medical devices under MDR: a brief guide
Clinical evaluation of medical devices is critical to obtaining and maintaining marketing approval in the EU. Learn more about clinical evaluation here.

EDQM and the CEP of the future
A certificate of suitability to the monographs of the European Pharmacopoeia (CEP) issued by the EDQM is considered to replace the relevant sections in the application for marketing authorization demonstrating the quality of substances for pharmaceutical use. The inclusion of a CEP in an MAA simplifies the review process for authorities and the submission procedures for industry. However, with the upcoming changes to the EU pharmaceutical legislation, the CEP procedure may be at risk. In this blog post, we outline the possible changes in the near future.
CAR-T cell therapy: main components, manufacturing, and prospects
CAR-T cell therapy uses genetically engineered T cells to destroy tumor cells. Learn more about the main components, manufacturing process and prospects of this promising immunotherapy.

10Y Dominiek Rossillion at QbD: from Quality Engineer to Business Development Manager
Dominiek Rossillion has been working for QbD for 10 years now, and counting! Our heartiest congratulations! Curious about Dominiek’s career and insights? Then be sure to read this interview!

What is EUDAMED and what new requirements should medical device companies take into account?
The new medical device regulations and the associated implementation of EUDAMED are intended to give the general public access to relevant information on medical devices, ensuring greater transparency. However, EUDAMED also brings new requirements for market participants throughout the life cycle of medical devices. Read more about them here!

GAMP categories for computerized systems: what are they and what are they for?
GAMP categories are very important to decide on the validation strategy of your computerized system. Learn more about all categories here.

10 questions for ‘10Y anniversary girl’ Katrien Janssens
10 years! That’s how long Katrien Janssens has been working at QbD now. Congratulations! Curious to know how Katrien has experienced the past decade at QbD? During a brief interview, we asked her 10 quick questions: one for each year she has worked with us!

3D printing medical devices: enabling patient-specific solutions for a better quality of care
3D printing medical devices offers unrivaled flexibility and personalization as illustrated by the case of Joe DiMeo’s face and hand transplant. Curious? Be sure to read on!

The key role of Regulatory Affairs in the pharmaceutical industry: from development to commercialization
Regulatory Affairs plays a key role in the pharmaceutical industry: from drug development to commercialization. Learn more about the roles and functions that RA teams can provide in the lifecycle management of your pharmaceutical product.

Quality Control challenges in closed systems: implementing AI as an in-process control
Sampling, testing, and preserving your medicinal product in closed systems often present difficult Quality Control challenges. As part of the solution, various AI-based in-process controls can be used. Discover them here!
Cell therapy as an ATMP: introduction, definition, and subtypes
Cell therapy includes various ATMPs to fight tumors or restore the physiological status of damaged tissue. Explore them here.

10 Questions for ‘10Y anniversary boy’ Jeroen Verhoeven
If you know that QbD was founded 10 years ago, then it won’t come as a surprise to you that the 10Y anniversary of an employee is an EXTRA big deal to us. After QbD founder Bart (obviously), Jeroen Verhoeven is the very first employee to reach that landmark.
In order to give this news the attention it deserves, we fired 10 questions at Jeroen, one for each year he’s been with us!

QbD and TRIUM awarded with Baanbrekende Werkgever ‘22 certificate
On November 15, 2021, QbD and TRIUM received the Baanbrekende Werkgever ‘22 certificate for pioneering Belgian employers striving to put people at the center of their policies on hybrid work and mobility.
Dendritic (DC) cells: effective sentinels for immunotherapies
Dendritic cells play an important role in recently developed DC-based immunotherapies for the treatment of cancer, autoimmune diseases and the prevention of graft rejection. Read all about DC cells and their potential in this article.
Natural Killer (NK) cells: a major breakthrough in the field of ATMPs
Natural Killer (NK) cells, as part of our innate immune system, are very effective in killing different types of cells, including tumor cells. Because of these properties, they become very interesting as ATMPs for immunotherapy.
Curious about the potential of Natural Killer (NK) cells and NK-cell-based immunotherapy? Learn all about them here, including the benefits and challenges.
From IVDD to IVDR: avoid these pitfalls to get your CE marking in time
In the EU, the IVDR will replace the IVDD. The compliance deadline is approaching, so act now to obtain CE marking of your IVD medical device in time.

Medical device combination products: what regulations should you apply before marketing them in the EU?
Follow these steps before launching medical device combination products in the EU, including the application of MDR Article 117 and the EMA Guidance.

IVDR classification of in-vitro diagnostic medical devices: a brief guide for manufacturers
Learn more about the new risk-based approach to in-vitro diagnostic device classification under IVDR 2017/746, replacing outdated directives.

MDR impact on MDSW: what has changed from MDD?
The introduction of MDR impacts CE-marked medical software (MDSW). Curious about the scope of this impact on your software? Read more below.
Medical Device Industry Trends: on wearables, AI, POC testing, IoT, and more
Curious about the latest medical device industry trends? Learn more on wearables, AI, POC testing, IoT, and more in this blog post!

QbD Academy welcomes 15 young graduates to jumpstart their careers in life sciences
The QbD Academy is a young graduates program that combines working, learning and intensive training. Last month, we welcomed 15 young graduates during our QbD Academy Week. Read all about it here!

Unraveling Artificial Intelligence in Medical Devices: what do we know so far?
Artificial Intelligence is the IT word in Medical Devices nowadays. Learn more on AI and its regulatory implications for manufacturers.

SaMD versus MDSW: what’s the difference between Software as a Medical Device and Medical Device SoftWare?
The terms SaMD (Software as a Medical Device) and MDSW (Medical Device SoftWare) are often used interchangeably within medical environments, but they are not. Learn the difference in meaning and regulatory scope here.

5 consequences of Brexit when selling medical devices in the UK
Brexit impacts the medical device markets of Scotland, England, Wales and Northern Ireland. Learn more on the consequences for manufacturers that place medical devices on the UK market here.

ATMPs: translating the expertise into a GMP process
ATMPs are a promising new type of medicine. Learn more about the challenges in translating the expertise into a GMP process.

What is a medical device? Key definitions and regulations around the world.
Is your product a medical device? Well, that depends on the markets you want to enter and their regulations. Here’s an overview to guide you.

Why Medical Device Risk Management is as complex as it is crucial
Risk management, the key to medical device safety, involves much more than ticking the FMEA box. Jeroen Verhoeven, one of our specialists in the matter, explains why and guides you through the ISO14971 standard (application of risk management to medical devices).

How QbD is investing in the new hybrid way of working
At QbD, we’re going the extra mile to put hybrid working to everyone’s advantage after COVID-19. Need some inspiration? Follow our lead!

Clinical Trials for ATMPs: which challenges to overcome?
ATMPs are, by nature, highly complex and innovative therapies. And well-designed clinical trials are key to producing effective and safe ATMPs – ultimately increasing patients’ health and speeding up market authorization. In this blogpost, Marie-Paule Gyselen from TRIUM Clinical Consulting (part of the QbD Group) guides you through the most important ATMP-specific clinical trial challenges.

From idea to post market surveillance: the phases of the medical device lifecycle
Launching a medical device requires much preparation. As these devices are heavily regulated, a company has to meet many requirements in different phases of the development and manufacturing process. Which phases are there to consider and what are their characteristics? This blog digs deeper into the lifecycle of a medical device and requirements that must be met in those phases.

10 things you should know before validating Computerized Systems
Not sure where to start when validating your computerized systems? Follow these 10 steps as a guideline.

Smart factories: How digital twins can strongly improve your ATMP manufacturing processes
In the smart factories that came with Industry 4.0, digital twins of manufacturing processes are key to improving process robustness, development lead times and ultimately reducing costs. In this blog Evan Claes and Tommy Heck from our partner Antleron explain how digital twins work and why they are so vital, especially to ATMP manufacturing.

Why QP challenges in ATMPs are different & what you need to know!
In this blog post, we give more insights into the challenges a QP faces when dealing with ATMPs. We talk about sterility, out-of-specification handling, batch release, and EU import testing.

How do you reduce the Cost of Goods, which is key to affordable ATMPs?
The sales price of cell and gene therapies is influenced by multiple factors like Cost of Goods (COG), regulatory requirements, target market (or commercial aspect), and the development just to name a few. In this blog post you will learn more about the GOC aspect: what is this cost comprised of and how can you reduce it?
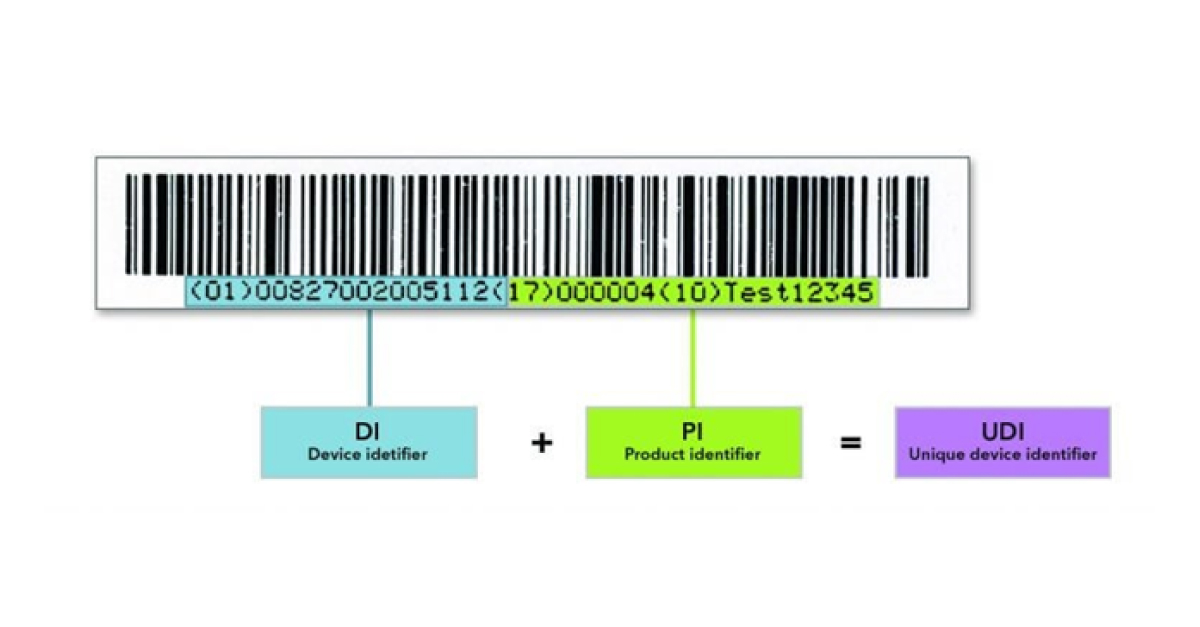
What you need to know to make your medical device UDI-ready in time
UDI is a new system that assigns a unique barcode to each individual medical device. It will become mandatory when MDR and IVDR enter into force, so as a manufacturer, you need to make sure you’re UDI-ready in time.

Post-Market Surveillance of Medical Devices
A short introduction and overview of the requirements In 2017, the European Commission released the new Medical Device Regulation (MDR) that will replace the Medical

Medical devices: innovations versus regulations
One of our previous blogposts discussed innovations in the medical technology field and how these can fulfill unmet patients’ and physicians’ needs by leveraging the
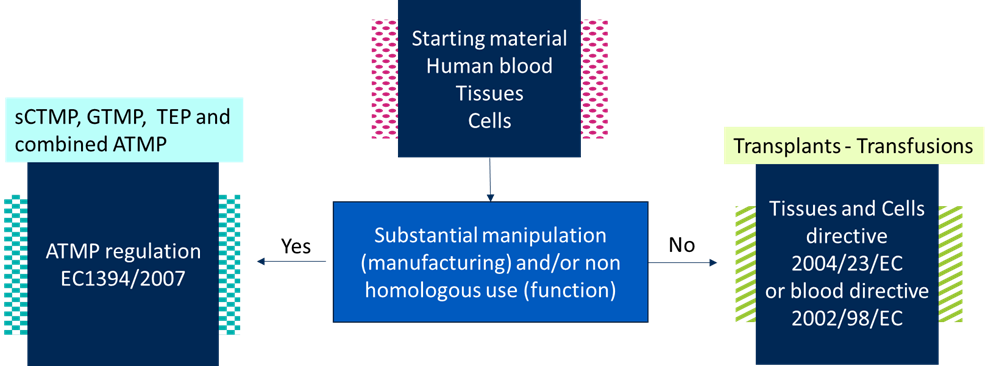
Is your ATMP platform robust enough to deal with material input variability?
Do you want to distinguish starting materials used for ATMPs from those used for transplants or transfusions? This distinction is made based on how the
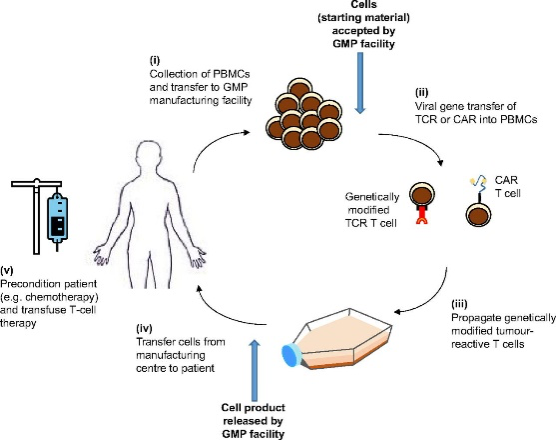
CAR T and TCRs for the market: current state and future perspectives
Among the Advanced Therapy Medicinal Products (ATMPs), in the immunotherapy market, nowadays two acronyms catch the eye: CAR-T and TCR-T. The two techniques are based
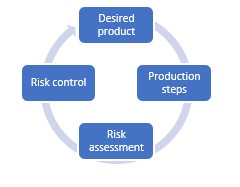
Quality by design methodology for the pharmaceutical industry
It’s obvious that product quality and patient safety are a top priority in the pharmaceutical industry. The quality by design methodology is used to build
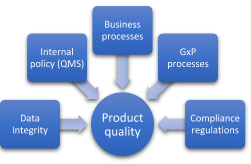
What to focus on when validating a computer system? (3)
GxP Processes Without question, the impact of GxP processes such as product quality and patient health are at the top of the list when it
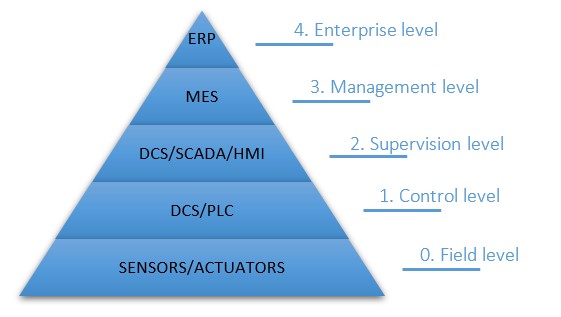
Automation Pyramid – FAQ
What does an automation pyramid entail? An automation pyramid visualises the integrated layers of technology used in manufacturing or industry combined with the level of

What to focus on when validating a computer system? (2)
In the first blog we discussed the definitions of validation and computerized systems. Checking the fitness of the system is to check whether the system operates according to the needs of the process for which it is acquired.

Three important trends in technology transfers
Have you read our earlier blogposts about the The importance of a dedicated team and skilled project manager for technology transfers, the factors that can make or break your technology transfer, and the drivers of complexity in tech transfers?

ATMP manufacturing for the market: main hurdles to be tackled to ensure a high-quality end product.
Until now, academia and research-based companies have been the major source of successful ATMP (gene therapy and cell- or tissue-engineered product) developments. Based on their success, pharmaceutical companies are trying

What to focus on when validating a computer system
In the first blog we discussed the definitions of validation and computerized systems. Checking the fitness of the system is to check whether the system operates according to the needs of the process for which it is acquired.

The importance of a dedicated team and skilled project manager for technology transfers
Have you read our earlier blogposts about the factors that can make or break your technology transfer, and the drivers of complexity in tech transfers?

How to start with computer system validation?
Life sciences enterprises in for example the pharmaceutical, biotechnology and medical devices industry must validate computer systems that have an impact on the product and

Tech transfer: what are the drivers of complexity?
In this blogpost, we will dive into these complexities a little bit more and provide you with some useful tips to minimise the complexity of your tech transfers.

Technology transfers: these factors can make or break them
A technology transfer can occur between buildings on the same production site, different production plants from the same company, or even between different companies. It
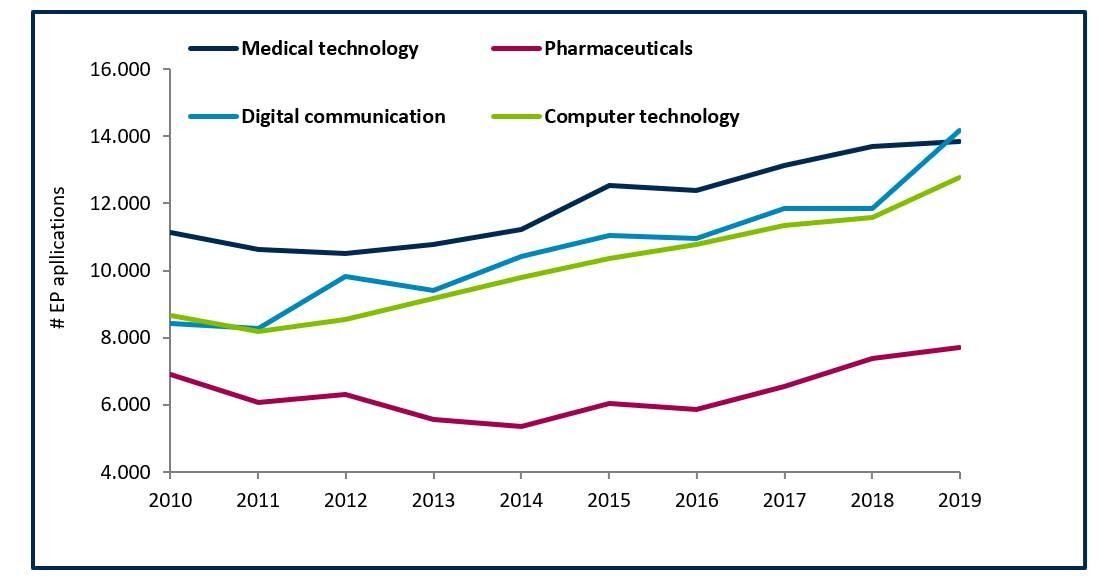
Innovations in Medical Technology: The Future of Healthcare is Data
Innovation is key to the success of companies and is driving many industries. This is certainly true for the medical technology sector. According to yearly

Starting a new job in Corona times: this is how Katrien experienced it at QbD
A hiring freeze during Corona? No way! Although the current crisis has created new and unexpected challenges, QbD is not the kind of company to

A Day in the QbD life of… Frederick Tison – Corona times
Hi! My name is Frederick Tison. I’m 26 years old and this is my third year at QbD. I’m currently active as a raw material
Anything you need to know about ATMPs: FAQ
What is the definition of ATMP? From the website of EMA: Advanced therapy medicinal products (ATMPs) are medicines for human use that are based on

ATMPs in a nutshell: what you should know about classification, quality and go to market
Advanced therapy medicinal products (ATMP) is the group of therapies in which we use cells, genes, engineered tissues and combinations of the above with medical

Artificial Intelligence in Advanced Therapy Medicinal Products
Advanced therapy medicinal products (ATMP) are cell and gene therapies that provide new opportunities for the treatment of a variety of diseases and injuries. Due

Computer Systems: is Assurance the new Validation?
The past of Computer Systems Validation has served us well and provided us with the foundation of where we are today. But at some point, the past can become a burden. This is exactly the point in time where we have arrived.

How will Brexit affect the pharmaceutical industry in European Union?
The clock is ticking for Brexit. By 31 October 2019, a final agreement on the matter must be reached. There is no doubt that the UK leaving the European Union (EU) will have an enormous impact on the UK and EU member states.
Market release of vaccines: European Union versus the United States of America
When it comes to the market release of vaccines, there are some specific requirements and legislations that companies need to keep in mind. In this blog post, we will pay attention to the market release of vaccines onto two of the most important markets worldwide: the European Union and the United States of America. Although they are quite similar, there are some interesting differences when it comes to testing, documentation and certification.

A day in the QbD life of… Julie, HR business partner at QbD HQ
It’s time again for a closer look into the daily life of another one of our QbD’ers! Meet Julie Bondroit, one of the members of

Technical Writing in Pharma and Biotech: Essential Tips
Discover key tips for effective technical writing in pharma & biotech. Learn the dos and don’ts to minimize errors and improve quality.

Serialization in the EU can become a competitive advantage if you dare to innovate
Counterfeiting has always been a significant issue when it comes to medicinal products. While it might sound like a good deal when you can find

EU GMP Annex I: Are we ready for the challenges posed after its revision?
EU GMP Annex I is a document published by the EC relating to the manufacture of sterile products. Read more about the requirements here.
Pioneering with Artificial Intelligence to make personalized cell therapy more accessible
Several scientific and clinical breakthroughs of our past decade show that Advanced Therapy Medicinal Products (ATMP) will revolutionize 21st century medicine. By using cells as

Updating Good Clinical Practice E6(R2)
GCP has been expanded with an addendum. What has changed? Let’s take an overall look at GCP before we answer that question.

Preparing for growing challenges in pharmaceutical serialization
Serialization is rapidly emerging in the pharmaceutical world. To fight counterfeit drugs, more and more governments demand serialization.

Antleron & QbD team up to create a landslide in healthcare
A whole new era in healthcare is coming closer every day. Thanks to the crossover of 3D printing and biology, medical solutions will be personalized

Data integrity in the pharma industry – a short introduction
Over the last couple of years, the data integrity topic has received more and more attention. Recently, the American Food and Drug Administration (FDA)

Eudralex Vol 4: New annex 15 – Qualification
Explore the 2015 updates in Eudralex Vol 4: New Annex 15, covering expanded process validation, QbD, and innovative pharmaceutical manufacturing approaches.