Compressed gas standards
The latest draft of Annex 1, Manufacture of Sterile Medicinal Products, introduced the concept of a “contamination control strategy” which limits particulate and microbial contamination and promotes process understanding, which means:
- A thorough understanding of potential sources of contamination.
- Regular trend analysis is performed, ensuring appropriate critical quality attributes of high-risk utilities.
- Gases and other high-risk utilities that come in direct contact with the product or primary container are of appropriate chemical, particulate and microbial quality.
Specifically, actions should be taken to ensure the sterility of process gases, including filtration through a sterilizing filter at the point where the gas is used in production, and sterilization of any subsequent piping or tubing.
Filtration should be part of batch standards, with certification guaranteed before release. Integrity testing should be performed for both critical and non-critical gas filters.
“When gases are used in the process, microbial monitoring of the gas should be performed periodically at the point of use.”
Although reference to compressed air sampling is made within EU and FDA GMPs, ISO 8573 sets out the general approach and requirements for compressed gases. The ISO standard consists of the following parts:
- Part 1: Contaminants and purity classes,
- Part 2: Test methods for aerosol oil content,
- Part 3: Test methods for measurement of humidity,
- Part 4: Test methods for solid particle content,
- Part 5: Test methods for oil vapor and organic solvent content,
- Part 6: Test methods for gaseous contaminant content,
- Part 7: Test method for viable microbiological contaminant content,
- Part 8: Test methods for solid particle content by mass concentration,
- Part 9: Test methods for liquid water content.
The purity classes are aligned with what is commercially available to remove these major contaminants.
The summary chart (Figure 1) below shows purity classes 0 through X for particles, water, and oil. The end user can then select a purity class for each contaminant based on either equipment installed or air quality required for a specific process or product.
There should be no confusion on what contaminants and limits need to be tested, because that decision was made when compressor system filtration and point-of-use filters were selected.
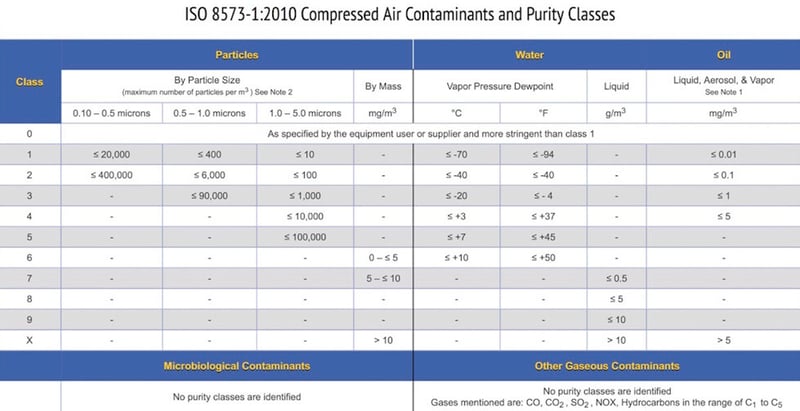
Figure 1 – ISO 8573-1:2010 Compressed Air Contaminants and Purity Classes
Note that ISO 8573-7, test method for viable microbiological contaminant content, does not specify the frequency or the limits for viable microbiological contaminants.
The frequency and limits should be based on a risk assessment of the activities and should be often enough to enable meaningful trends to be assessed.
Acceptable microbial numbers are based on an interpretation of GMP, like the FDA Aseptic Filling Guidance document (2004), which states:
“A compressed gas should be of appropriate purity (e.g., free from oil) and its microbiological and particle quality after filtration should be equal to or better than that of the air in the environment into which the gas is introduced.”
Microbiological contaminants in compressed gases
Compressed gas and air systems are relatively harsh environments, nevertheless microbial survival is possible, if there are nutrients available.
Nutrients suitable for metabolizing by microorganisms include water and oil droplets. Another factor that can affect survival is temperature.
In addition to vegetative cells, bacterial spores are well equipped to survive the harsh environmental conditions. Spores are resistant to the types of temperature ranges and moisture levels found within compress gas lines.
Another risk exists with biofilm, where microbial communities can potentially form and develop through attachment to air lines and tubing.
Typically no microorganisms would be expected to be recovered from compressed gas lines. More often the source is adventitious contamination.
Sources of contamination include:
- Source of the air of gas. With air, this is air intake air from surroundings (which can contain oil, dirt/dust and moisture/ water vapor, microorganisms).
- Piping distribution systems. Piping distribution and air storage tanks, more prevalent in older systems, will have contaminants in the form of rust, pipe scale, mineral deposits, in addition to bacteria.
- Bacterial retentive filter. The filter may become blocked, lose its integrity, or become wet.
- Compressor failure. The compressor itself can create a contaminated For example; the compressor’s pre-filters can become overloaded with dust and lint, causing the filter to cease functioning properly.
- Sample valve. The point-of-use sample valve may not be designed correctly or become faulty.
Compressed gas microbial monitoring plan
Analyzing micro burden data at point of use outlets throughout compressed air pipeline systems at a given time, acts as a window of observation into the control of the facility.
Maintaining control means proper preventative maintenance, microbial monitoring scheduling and risk assessment must be appropriate for the industry being monitored.
Once the compressed air microbial monitoring plan is approved, a sampling procedure that provides the company with the results suitable to its limits and specifications needs to be established.
This requires the use of a procedure that accurately measures and samples a specific volume of air for microbial burden analysis inside the tested compressed air system.
Compressed air sampling should form part of an environmental monitoring program, along with cleanroom assessments.
The program should take into account air points to be tested. This could be every point; points considered to be of greater risk (such as product contact); or representative points along a loop.
The frequency of testing must also be considered, and this
too would need to tie into risk.
Why use a contract Lab?
Investment in the appropriate equipment can be expensive, not only in terms of the cost of the analytical equipment, but also the preparation capability, infrastructure such as gases and extraction, as well as analyst training and qualification of the instrumentation.
The use of an accredited contract analysis laboratory is often a highly effective way of carrying out validations and routine elemental analysis. Benefits include
- Speed of analysis
- Knowledge
- Clear view on costing upfront
Quercus, your trusted GMP Laboratory
Quercus Laboratories is a member of the QbD group and is a trusted GMP Laboratory that supports the pharmaceutical industry with a wide range of QC testing services. We offer a complete service from chemical and microbiological QC testing of raw materials and finished drug products.
Our capabilities include:
Analytical Method Validation – Batch release – EU Import services – Routine QC Batch Analysis – Raw material testing – Microbiological testing – Preservative Efficacy Testing – Stability Studies and Storage – Clean Room Validation – Active Air Sampling – Compressed Gasses Monitoring – Elemental Impurity Testing – Endotoxine Testing – Purified Water Analysis (TOC) – Cleaning Validation
References
- USP 1116 – Microbiological evaluation of clean rooms and other controlled environments
- ISO 8573 – Compressed air
- EU GMP – Annex 1: Manufacture of Sterile Medicinal Products
- ICH Q9 Quality Risk Management







.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)





