Dendritic cells (DCs) are crucial cells in the initiation and regulation of the adaptive immune response. These leukocytes form a link between the innate and adaptive immunity. They are also called antigen-presenting cells (APCs) since they are responsible for capturing, processing and presenting antigens of detected pathogens to lymphocytes.
Their main responsibility is recognizing pathogens and attacking them with the help of Natural Killer (NK) cells. DCs express distinct patterns of cytokines to attract T cells for more effective action against pathogens. This will activate naïve T cells and differentiate them into effector T cells.
Immunotherapies in which dendritic cells are manipulated hold great promise for cancer treatment, prevention of transplant rejection and autoimmune diseases.
This blog will provide an introduction to the function of dendritic cells and outline their important role in immunotherapies, with a focus on cancer treatment.
Characteristics of dendritic cells
Development of dendritic cells
DCs have a tree-like shape and are also called the “sentinels” of the immune system. They are derived, along with macrophages, from the myeloid progenitor “macrophage-dendritic cell progenitor (MDP)” in the bone marrow.
Macrophages are white blood cells that engulf and digest cells by phagocytosis. From MDP, DC progenitors (pDCs) arise, which then transform into their major (sub)types.
The figure below shows the simplified pathway of the development of dendritic cells.

Figure 1 – Development of dendritic cells. (Adopted from https://europepmc.org/article/med/25999945)
The function of dendritic cells
DC’s contain unique Pattern Recognition Receptors (PRR), which can recognize different Pathogen Associated Molecular Patterns (PAMPs) that are present on pathogens. As such, DC’s present antigens of foreign cells (i.e. bacteria) on their surface for faster recognition of other immune cells.
DC’s migrate towards the regional lymph nodes, where they stimulate the activation and proliferation of antigen-specific T and B lymphocytes. Further, DC’s interact with natural killer cells, which have a crucial role in combatting viral infections.
Dendritic Cell Immunotherapy
The development of cancer immunotherapy has been a long time coming. Many years have been invested in researching suitable chemotherapeutics, antibodies, and eventually ATMPs that could treat cancer and solid tumors.

Figure 2 – Dendritic Cell Immunotherapy. Adopted from: Stem Cell Treatment for Cancer in Mexico | Dendritic Cells Immunotherapy (stemcellmexico.org)
When DC therapy is being produced, DCs are collected and matured ex vivo to bypass the initial immunosuppressive influence of the tumor microenvironment (TME) and tumor cells on endogenous DC maturation.
Antigens of tumor cells are presented on the surface of DCs during their maturation. Because of these antigens, tumor cells are attracted to the DCs, which enables them to recognize the type of tumor cell itself.
After choosing and multiplying the DCs, they are injected back into the body of the patient where the DCs will present the antigen to the T cells. By presenting these antigens, T cells will evolve into effector T cells. These effector T cells will then have different functions, such as killing the tumor cells. But also supporting other T cells and building up and supporting the immune memory against these tumor cells.
An extra advantage of administrating autologous DCs could be that the in vivo tumor-specific immune response is induced and even improved.
Dendritic cell therapy challenges
Dendritic cell therapy is only effective in a limited number of patients and depends on the nature and type of malignancy the patient is dealing with. The choice of antigen(s), the method of loading, and the type of DCs used are also dependent factors on the effectiveness of the therapy.
Another challenge is that tumor cells may have immunosuppressive actions against the DCs and T cells, preventing them from performing their function. If the tumor has a tumor microenvironment (TME), which is usually composed of connective tissue, it may also impede the immune-activating potential of the administered DCs and suppress the function and infiltration of activated T cells.
For these various challenges, research is being conducted to address these immunosuppressive features. This is being done by combining DC therapy with other approved therapies such as chemotherapy, radiotherapy, and immune checkpoint blockers. The goal of these combinations is to reduce the immunosuppressive effect of tumor cells and improve DC therapy so that it can be used in more patients and in more types of tumor treatments.
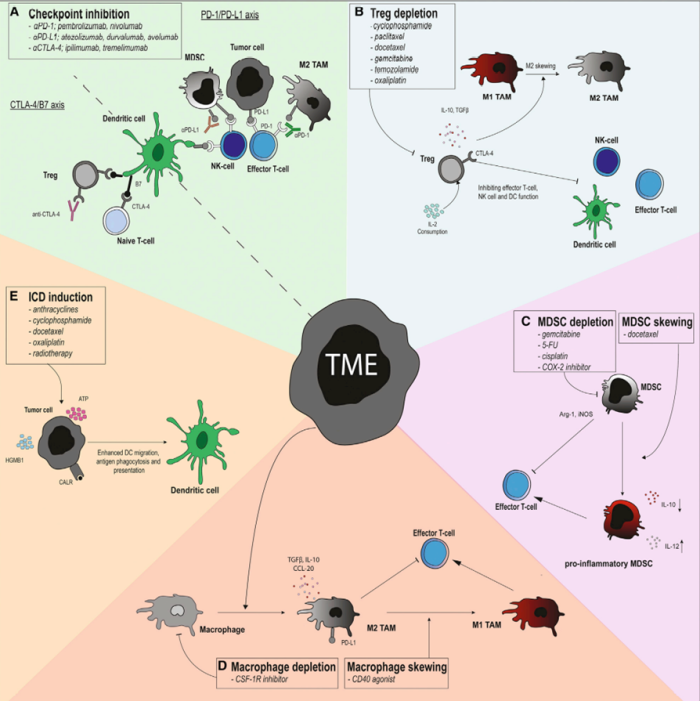
Figure 3 – Different techniques combined with DC Therapy to modulate the effects by the TME. Adopted from: Enhancing Dendritic Cell Therapy in Solid Tumors with Immunomodulating Conventional Treatment: Molecular Therapy – Oncolytics.
Conclusion: DCs are important sentinels for immunotherapies
Dendritic cells (DCs) are a very important link between the innate and adaptive immune systems as antigen-presenting cells (APCs). By presenting antigens in lymph nodes, they can activate naïve B and T cells into effector B and T cells who will attack and kill tumor cells and also build up and support the immune memory against these specific tumor cells.
People with specific types of tumors can be treated with DC therapy. By collecting DCs, maturing and multiplying them with specific antigens of the tumor, and injecting them back into the patient, the DCs can support the adaptive immune system by rapidly activating the T and B effector cells which results in killing the tumor cells.
Although only a limited number of people can benefit from these therapies, researchers are trying to combine them with various types of other therapies, such as chemotherapy, radiotherapy, or immune blockers. The main reason for this is that more people can benefit from these therapies and require less therapy with serious side effects.
Do you need help with ATMP-related problems? Do not hesitate to contact our ATMP experts. We’d be happy to assist you!







.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)










.png)

.jpg)
