Medical Devices
Are you looking for quality, regulatory or clinical support for your medical device? QbD Group can support you at any stage in the lifecycle of your medical device, from idea to patient.
We are experts when it comes to ISO13485, clinical evaluation/investigation, MDR, design and development, technical files, auditing, qualification, validation, risk management, software, and more.
Regulatory expertise you can trust
With in-depth knowledge of ISO 13485, MDR, and risk management, we help you navigate complex regulations to safely and effectively bring your device to market.
Comprehensive quality and clinical solutions
From establishing quality management systems to conducting clinical evaluations and investigations, QbD Group ensures your medical device meets the highest standards of safety and performance.
Post-market monitoring and maintenance
We support ongoing compliance by managing post-market monitoring and processes to maintain the safety and effectiveness of your medical device throughout its lifecycle.
We cover the full Medical Device life cycle
From regulatory expertise to quality assurance and clinical evidence, we partner with you to accelerate your journey from idea to patient.
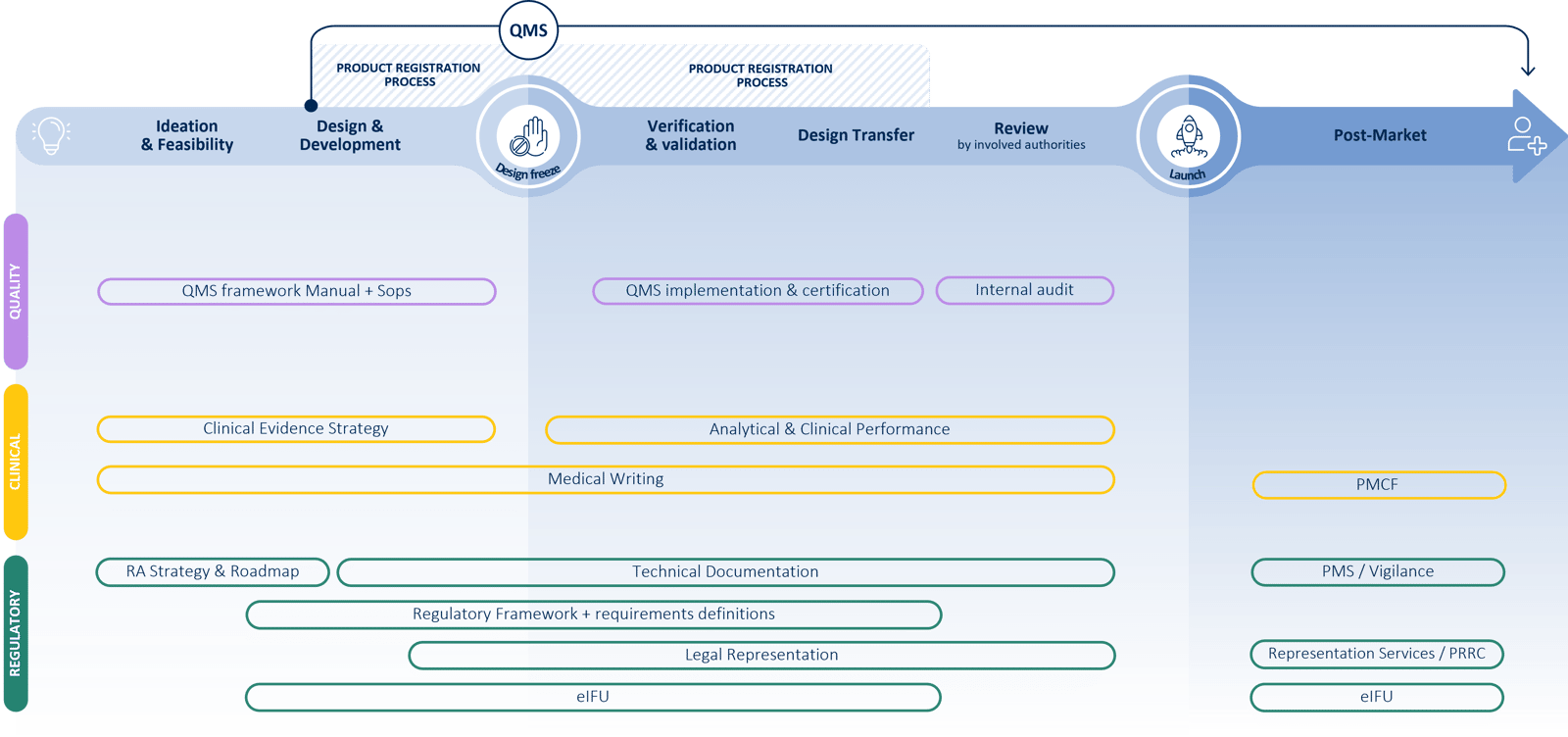
How we can help you
QbD Group offers a comprehensive suite of services tailored to address the unique needs of medical device manufacturers.
Related offerings
Industry challenges
Medical devices are regulated by laws that govern the safety and performance of devices across their lifetime, from pre- to post-market. A risk-based classification system determines the requirements for medical devices.
Medical devices with higher risks for the users require Notified Bodies to perform assessments on the safety and performance of the device. In this way, they ensure the continued safety and performance of medical devices, pre-, and post-market.
Medical devices with higher risks for the users require Notified Bodies to perform assessments on the safety and performance of the device. In this way, they ensure the continued safety and performance of medical devices, pre-, and post-market.
Manufacturers must ensure to only place medical devices in the market that comply with the legislative requirements.
This requires documentation of the design, development, and manufacturing process of the device including sufficient clinical evidence that shows the device is safe and performs as intended.
By establishing a quality management system and documented procedures for manufacturing and post-market monitoring activities, the quality of the product is maintained leading to continued safety and performance of the medical device during its lifecycle.
By establishing a quality management system and documented procedures for manufacturing and post-market monitoring activities, the quality of the product is maintained leading to continued safety and performance of the medical device during its lifecycle.
.jpg?width=1600&height=900&name=Medical%20Devices%20%20Our%20Industries%20-%20QbD%20Group%20(1).jpg)
Industry challenges
Medical devices are regulated by laws that govern the safety and performance of devices across their lifetime, from pre- to post-market. A risk-based classification system determines the requirements for medical devices.
Medical devices with higher risks for the users require Notified Bodies to perform assessments on the safety and performance of the device. In this way, they ensure the continued safety and performance of medical devices, pre-, and post-market.
Medical devices with higher risks for the users require Notified Bodies to perform assessments on the safety and performance of the device. In this way, they ensure the continued safety and performance of medical devices, pre-, and post-market.
Manufacturers must ensure to only place medical devices in the market that comply with the legislative requirements.
This requires documentation of the design, development, and manufacturing process of the device including sufficient clinical evidence that shows the device is safe and performs as intended.
By establishing a quality management system and documented procedures for manufacturing and post-market monitoring activities, the quality of the product is maintained leading to continued safety and performance of the medical device during its lifecycle.
By establishing a quality management system and documented procedures for manufacturing and post-market monitoring activities, the quality of the product is maintained leading to continued safety and performance of the medical device during its lifecycle.
Our offerings for Medical Devices in
.jpg)
.jpg)











.jpg)



.jpg)

.jpg)


.jpg)

-1.jpg)

-2.jpg)

.jpg)

.jpg)
.jpg)
.jpg)
.jpg)

.jpg)









.jpg)
Why QbD Group?
YOUR MEDICAL DEVICES INDUSTRY EXPERT
Within QbD Group, we have the knowledge and expertise to support manufacturers, distributors, and importers of medical devices in complying with the legislative requirements during the complete lifecycle of a medical device.
Our clinical, regulatory, and quality experts know what needs to be done to be able to comply with the legislative requirements by which a medical device can be safely placed on the market.
Furthermore, we keep supporting businesses by managing the processes that ensure the medical device continues to be safe and effective, before and after placing it on the market.
10+ years of experience
Full lifecycle support
Global presence
Best managed company

Get in touch
Partner with QbD Group to ensure your medical device meets the highest standards of quality and compliance. Fill out the form and our experts will help you navigate the development process with ease and efficiency.
Resources

Whitepaper

Whitepaper

Case study

Whitepaper

Whitepaper

Whitepaper

Case study
%20Checklist.jpg)
Whitepaper

Webinar

Whitepaper

Webinar

Whitepaper

Webinar

Whitepaper

Whitepaper

Whitepaper

Case study

Webinar

Whitepaper
.jpg)
Webinar

Whitepaper

Webinar

Whitepaper

Webinar

Webinar

Whitepaper

Whitepaper
.jpg)
Webinar

Whitepaper

Case study
Ensuring a smooth MDR transition for Oystershell's medical devices
The landscape of medical device regulations is constantly evolving, making it a challenge for companies to keep up. Our collaboration with Oystershell, which began in July 2023, highlights our expertise in transforming complex challenges into success stories, all with a touch of QbD Clinical magic.

Case study
.jpg)
Webinar
.jpg)
Case study

Case study
.jpg)
Case study

Case study
.png)
Case study

Blog

Blog

Blog

Blog
Get the latest industry news
Staying on top of the latest in the life science industry can be a daunting task. This newsletter will keep you up-to-date with the latest news, blogs and webinars so you can keep ahead of the curve.
Subscribe on LinkedIn

Connect with us at these events
Industry
Service
July
August
September
October
November
No Events Found.
Load More








.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)



-1.jpg?width=540&height=300&name=External%20newsletters%20rechthoek%20(2)-1.jpg)



