Cell therapy, a key component of Advanced Therapy Medicinal Products (ATMPs), represents a groundbreaking approach in modern medicine. Defined by the EMA as medicines for human use based on genes, cells, or tissue engineering, ATMPs stand at the forefront of treating various diseases and injuries.
As illustrated in figure 1, ATMPs are categorized into gene therapy products, somatic cell therapy products, and tissue-engineered products, with a fourth category combining ATMPs and medical devices (combined ATMPs).
This blog will delve into several ATMP-related cell therapies, particularly highlighting their role as some of the most promising breakthroughs in cancer treatment.
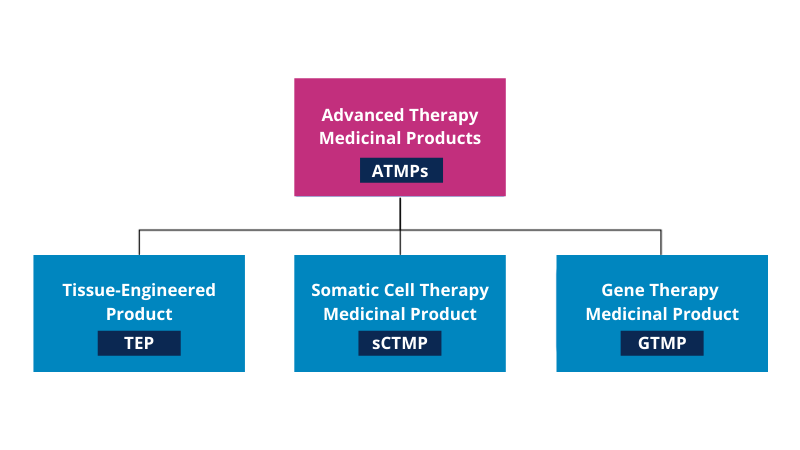
Figure 1 – Schematic overview of branches within ATMP
What is cell therapy?
Cell therapy includes all forms of treatment in which body cells are used to heal or support the patient. The cells are manipulated or edited to change certain biological characteristics before being transferred to the patient.
- When these cells come from the same person (donor = patient), we speak of autologous cell therapy.
- In allogeneic cell therapy, cells from another person (donor) are processed.
- And, as a third option, xenogeneic cell therapy uses animal cells.
Composition of blood – blood cells
The composition of blood can be defined as half plasma and half-blood cells (including red blood cells, white blood cells, and platelets). These blood cells are produced in the bone marrow and are formed from hematopoietic stem cells (first stage blood cells).
- The red blood cells or erythrocytes are the main component of the blood cells and act as oxygen carriers from the lungs to the rest of the body.
- Then there are the white blood cells or leukocytes, which fight infections and participate in the immune response.
- Finally, there are the platelets or thrombocytes, which help with blood clotting.
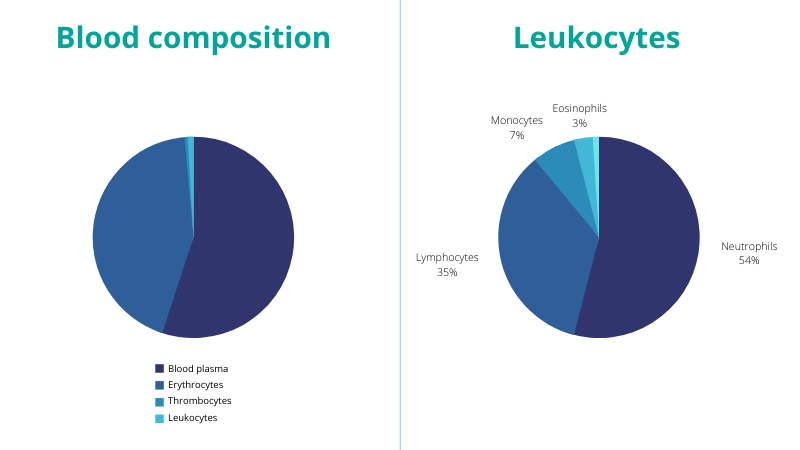
Figure 2 – Blood composition
The leukocytes can be further distinguished in granulocytes and non-granulocytes. 5 subtypes are defined:
- Lymphocytes (non-granulocyte)
- Monocytes (non-granulocyte)
- Eosinophils (granulocyte)
- Basophils (granulocyte)
- Neutrophils (granulocyte)
The two main types of lymphocytes are T lymphocytes (T cells) and B lymphocytes (B cells) and a third example of lymphocytes are the Natural Killer (NK) cells. These leukocytes are the cells that qualify for cell therapy because they are active in the immune response against infections. Within the mononuclear phagocytes, it is the dendritic cells that are eligible for cell therapy, while monocytes and macrophages are not.
Cells used for ATMP: current ATMP-related cell therapies on the market
Of the blood cells described above, T cells, B cells, DC cells, and NK cells are used in the manufacture of ATMPs in the context of cell therapy, while other, non-blood cells are used in the context of tissue engineering products.
The following sections provide an overview of the different klinds of cell therapy used today. Cell therapies based on T-lymphocytes, B-lymphocytes, Dendritic cells (DC), and Natural Killer (NK) cells will be discussed respectively. A small intro to current tissue engineering products will also be given.
1. T lymphocytes
T lymphocytes are used in different types of cell therapy including Tumor Infiltrating-lymphocyte (TIL), Chimeric Antigen Receptor T-cell (CAR-T), and Engineered T-cell Receptor (TCR) therapy.
In TIL therapy, T lymphocytes are isolated within or near the patient’s tumors. These T lymphocytes are the ones that best recognize the tumor cells. They are expanded in the laboratory and finally reinfused into the patient. In this way, the patient receives a large amount of selected T lymphocytes that actively detect and destroy tumors.
TIL therapy is still in the clinical trial phase (the current phase is phase II) and has the greatest emphasis on the treatment of melanoma.
CAR-T and TCR therapy have already been discussed in a dedicated blog post and use the same general principle: T lymphocytes, extracted from the patient, are modified by the addition of a gene encoding for a specific receptor, generating CAR T-cells and TCR T-cells respectively. As a result, these cells possess the ability to detect and destroy malignant target cells.
The difference between the two techniques lies in the fact that TCR-T cells can only target malignant cells whose antigens are present on the surface of a major histocompatibility complex or MHC, whereas CAR-T cells can bind to antigens presented on the surface of the tumor cells.
This difference is the reason why CAR-Ts are more effective against blood tumors while TCR-Ts are better suited against solid tumors.
Multiple CAR-T cell therapies have been approved by the FDA and the EMA for the treatment of multiple cancers and patients with lymphoma or leukemia (Tecartus received a conditional license from the EMA on December 14, 2020); while TCR-Ts are in clinical trials phases I, I/II and II.
2. B lymphocytes
Next to T lymphocytes, B lymphocytes are also used in cell therapy. While T-lymphocytes help regulate the function of other immune cells and directly attack various infected cells and tumors, B-lymphocytes make antibodies, which are proteins that specifically target bacteria, viruses, and other foreign materials.
In addition to antibody production, studies are also underway to determine whether B lymphocytes can be used in cancer immunotherapy. For example, it is currently being investigated whether CD40-activated B cells can serve as a cellular cancer vaccine. To date, no cell-based therapy based on B lymphocytes has been approved by the FDA.
3. DC (Dendritic Cells)
Dendritic Cells (DCs) are considered professional antigen-presenting cells (APCs) that play a crucial role in tumor immunotherapy. They recognize pathogens and present them to the patient’s immune system.
In DC therapy, the dendritic cells are extracted/filtered from the patient’s blood and during maturation, the antigens of the tumor are presented, making the DCs capable of recognizing the tumor cells within a week.
The “trained” DCs are then reintroduced into the patient’s body by injection or infusion, strengthening the patient’s immune system against the target. They activate the cytotoxic T cells in the body to bind with the cancer cells, causing them to die.
This dendritic cell therapy is an approved therapy that was first used in the US in 2010 to treat prostate cancer. Be sure to check out our blog post on dendritic cells here.
4. Natural Killer (NK) Cells
Natural killer (NK) cells are large lymphocytes derived from pluripotent hematopoietic stem cells located in the bone marrow that provide an attractive and safe source of allogeneic cells for immunotherapy. They are the body’s first-line defense mechanism against pathogens (such as viral cells and cancer cells).
NK cells are immune effector cell types that defend against various pathogens (viruses such as Chlamydia spp., Rickettsia spp., Listeria monocytogenes, and Protozoa) that cause intracellular direct infections (both cytoplasmic and vesicular).
More specifically, NK cells are activated by interferons (proteins produced and released in response to infection) and, with their invariant activating and inhibitory receptors, destroy infected virus cells, some tumor cells, and other intracellular pathogens.
NK cell receptors can act as activators or inhibitors when a specific cellular ligand is recognized. At the time of recognition, the NK cell receptors will send a positive or negative signal, respectively, after which the tumor cells can be targeted.
The immune response exhibited by NK cells is threefold: either a cytotoxic immune response, an effector immune response, or an inhibitory action. A more in-depth discussion of NK cells can be found in an earlier blog (“Why NK Cells are a Major Breakthrough in ATMPs“).
To improve the recognition and destruction of tumor cells, NK cells are genetically engineered to express different types of receptors. One type of receptor is the chimeric antigen receptor (CAR), which results in CAR NK cells capable of targeting a very specific type of (tumor) cells/tissues or supporting NK cell function and survival.
Because of its great potential for the treatment of hematological malignancies, as well as for the treatment of solid tumors, CAR NK-cell-based immunotherapies are currently being investigated in clinical trials worldwide, but approved immunotherapy is not yet available.
5. Tissue Engineering
Tissue engineering refers to combining cells and biologically active molecules with scaffolds to form functional tissues. The scaffold acts as a structure (made of natural or synthetic polymers) that supports the growth and differentiation of cells and leads to a tissue-like structure.
The goal is to generate functional constructs (3D functional tissues) that can replace tissue or can be used to maintain or improve damaged tissues. Currently, known approved examples are artificial cartilage and skin.
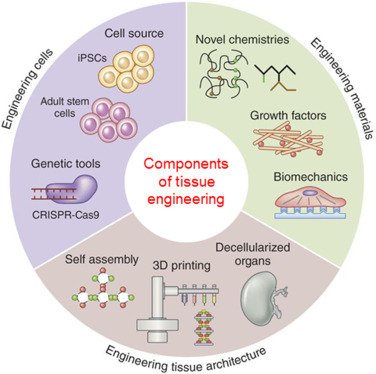
Figure 3 – Components of tissue engineering
The cells that are used in tissue engineering can be defined in three major cell sources:
- Embryonic cells (ES cells) and equivalent embryonic germ cells (EG cells)
- Autologous or allogeneic adult cells
- iPSC (Induced pluripotent stem cells)
Mesenchymal stem cells (MSC)
Mesenchymal stem cells (MSC) are adult stem cells that can be isolated primarily from bone marrow and adipose tissue, can proliferate to many generations, and possess the ability to differentiate into mainly osteogenic, chondrogenic, and adipogenic cell lines. They can be used in the healing process of tissue defects.
Autologous chondrocytes
Autologous chondrocytes can be combined with biomimetic cartilage scaffolds, resulting in in vitro tissue-engineered cartilage that can then be transplanted into patients to repair damaged cartilage.
This treatment is known as matrix-induced autologous chondrocyte implantation (MACI), which has been used since 2000. However, since 2014, the EU plant’s license for this advanced therapy drug MACI has been revoked, as the marketing authorization holder has closed this EU plant. This means that this therapy is currently not available to new patients in the EU until a new manufacturing site is registered in the EU.
iPSC
iPSCs are adult somatic cells, derived from the skin or blood, that are genetically reprogrammed into embryonic-like pluripotent cells. This allows for an unlimited source of all types of cells for use in tissue engineering.
Holoclar is a tissue engineering product approved by the EMA. Autologous cells from the limbus are taken and grown on scaffolds to form corneal cells. These tissue engineered cells are then implanted into the patient’s eye, where they will replace the damaged corneal surface.
Conclusion: cell therapy shows promise in different medical fields
Cell therapy includes various ATMPs in which cells, derived from autologous, allogeneic, or xenogeneic sources, are manipulated after which they are administered (via infusion or injection) to the patient. Tissue engineering offers the possibility of creating functional constructs that can replace a tissue.
The most commonly used cells in cell therapy treatments are T cells, B cells, NK cells, and DCs. This is one of the most promising treatments against several tumors, so we need to further explore and study this branch of health care.
Want to know more about this topic, or ATMPs in general? Contact the ATMP team at QbD to assist you with any ATMP-related issues.
- Advanced therapy medicinal products: Overview | European Medicines Agency (europa.eu)
- White blood cell count – Complete Blood Count (rnceus.com)
- Utilizing Xenogeneic Cells As a Therapeutic Agent for Treating Diseases (sagepub.com)
- ATMP – What are ATMPs? (atmpsweden.se)
- What are ATMPs 170620 (atmpsweden.se)
- Types of Stem Cell and Bone Marrow Transplants (cancer.org)
- Overview of Blood and Blood Components – Health Encyclopedia – University of Rochester Medical Center
- Gene & Cell Therapy FAQs | ASGCT – American Society of Gene & Cell Therapy | ASGCT – American Society of Gene & Cell Therapy
- Introduction to Stem Cell Therapy (nih.gov)
- Hematology Glossary – Hematology.org
- Dendritic cell biology and its role in tumor immunotherapy – PubMed (nih.gov)
- Dendritic Cell Therapy | Treatments | Infusio.org
- Components of blood (article) | Khan Academy
- CAR T and TCRs for the market: current state and future perspectives – Quality by Design (qbd.eu)
- T-cell Transfer Therapy – Immunotherapy – National Cancer Institute
- Adoptive Cell Therapy: CAR T, TCR, TIL, NK – Cancer Research Institute (CRI)
- CAR T-cell Therapy and Its Side Effects (cancer.org)
- Adoptive cell therapy using engineered natural killer cells | Bone Marrow Transplantation (nature.com)
- Perspectives of tumor-infiltrating lymphocyte treatment in solid tumors | BMC Medicine | Full Text (biomedcentral.com)
- B Cell-Based Cancer Immunotherapy – FullText – Transfusion Medicine and Hemotherapy 2019, Vol. 46, No. 1 – Karger Publishers
- Current status and perspective of CAR-T and CAR-NK cell therapy trials in Germany | Gene Therapy (nature.com)
- Immunotherapy Using Tumor Infiltrating Lymphocytes for Patients With Metastatic Melanoma – Full Text View – ClinicalTrials.gov
- Blood differential test: MedlinePlus Medical Encyclopedia
- Autologous-cell-derived, tissue-engineered cartilage for repairing articular cartilage lesions in the knee: study protocol for a randomized controlled trial | Trials | Full Text (biomedcentral.com)
- Overview of Tissue Engineering (verywellhealth.com)
- Principles of Tissue Engineering – Google Boeken
- Mesenchymal Stem Cells and Tissue Engineering (nih.gov)
- Induced Pluripotent Stem Cells (iPS) | UCLA Broad Stem Cell Center
- Matrix-induced autologous chondrocyte implantation (MACI): biological and histological assessment – PubMed (nih.gov)
- Maci | European Medicines Agency (europa.eu)
- Holoclar | European Medicines Agency (europa.eu)Patterns of dendritic cell and monocyte subsets are associated with disease severity and mortality in liver cirrhosis patients | Scientific Reports (nature.com)