Project Management & Support
QbD takes care of your short and long term projects. We deliver clearly defined results within a specific time frame and budget.
Find out more below.
Tailored project management and support
to address unique challenges
First and foremost, we listen very thoroughly to your needs and challenges. Do you want to implement a Quality Management System, or upgrade from ISO 9001 to a higher standard like ISO 13485? Or do you want us to carry out all your re-qualifications? Whatever your question, QbD understands that every challenge and client is unique.
That’s why we always start with a visit. This allows us to determine the scope of the project. This visit includes a design study, which consists of an interview with various stakeholders, and an audit that tells us to what extent you currently comply with existing standards and procedures.
The result is a detailed report, which includes all the steps necessary to meet your needs. This report is then translated into a plan with budgets, timelines, and roles.
If you decide to implement the above plan with our help, QbD offers two options depending on the size of the project and your own available firepower: consulting/coaching or an on-site QbD team executing your project.


Consulting and coaching
Depending on the outcome of our audit, we can support you with advice and coaching. In the case of consulting and coaching, one of our senior experts will support your organization. In addition, you will have access to our internal operational resources.

On-site QbD team to execute your project
If we fully pick up the project, execution consists of a kick-off meeting, weekly working committees and monthly steering committees. You will be supported by a dedicated team, including a senior project manager and a team of junior engineers.
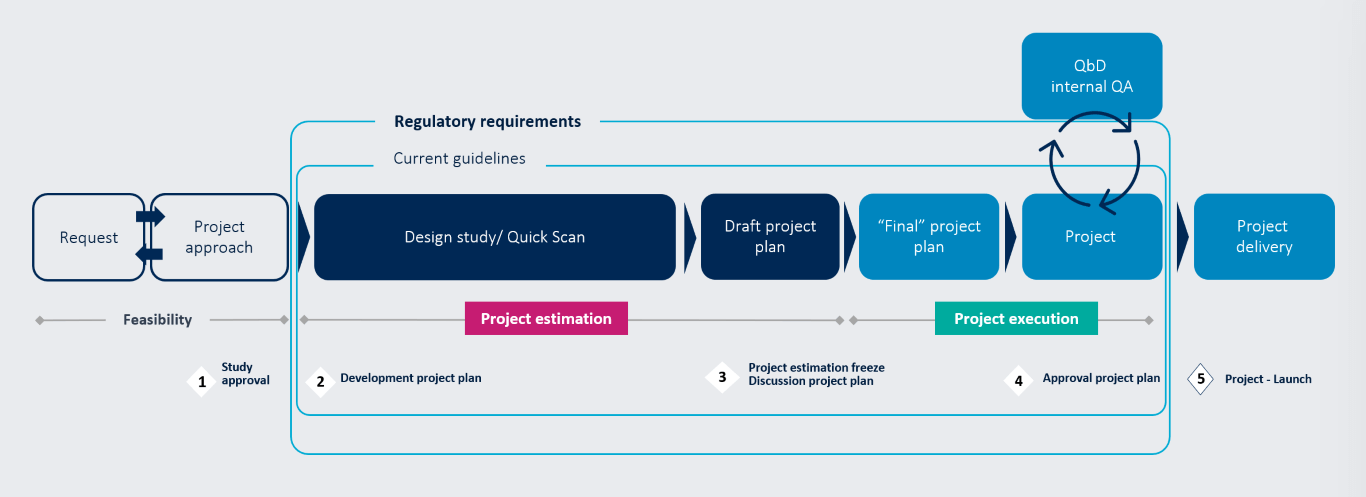
Overview of our project approach
Project estimation phase
Design Study
- Strategic high-level analysis of as – is and to – be
- Deliverable: Detailed project plan with effort estimation
Project execution phase
- Project initiation & execution after project plan approval
- Ongoing project verification with weekly working committees and monthly steering committees
- Self – steering team: Internal QA for continuous project improvement & documentation quality
Why QbD?
QbD supports companies worldwide in the life sciences throughout the entire product lifecycle, from idea to patient.
With more than 400 quality experts, QbD is your partner for advice and support.
Want to know how we would tackle your challenge? Or are you interested in a design study? Then don’t hesitate to contact us!
+10 years experience
Full cycle support
Global presence
Best Managed Company