Regulatory Affairs for In Vitro Diagnostics (IVD)
Are you looking for quality, regulatory or clinical support for your In Vitro Diagnostic medical device? The QbD Group by its affiliate Qarad can support you at any stage in the lifecycle of your IVD, from idea to patient.
We are experts when it comes to ISO13485, scientific validity, analytical and clinical performance, including performance evaluation studies evaluation, IVDR, design and development, technical files, auditing, qualification, validation, risk management, and software.
Discover more about what QbD can mean for your medical devices below.

Part of the QbD Group
This service is supported by Qarad
Challenges in In Vitro Diagnostics
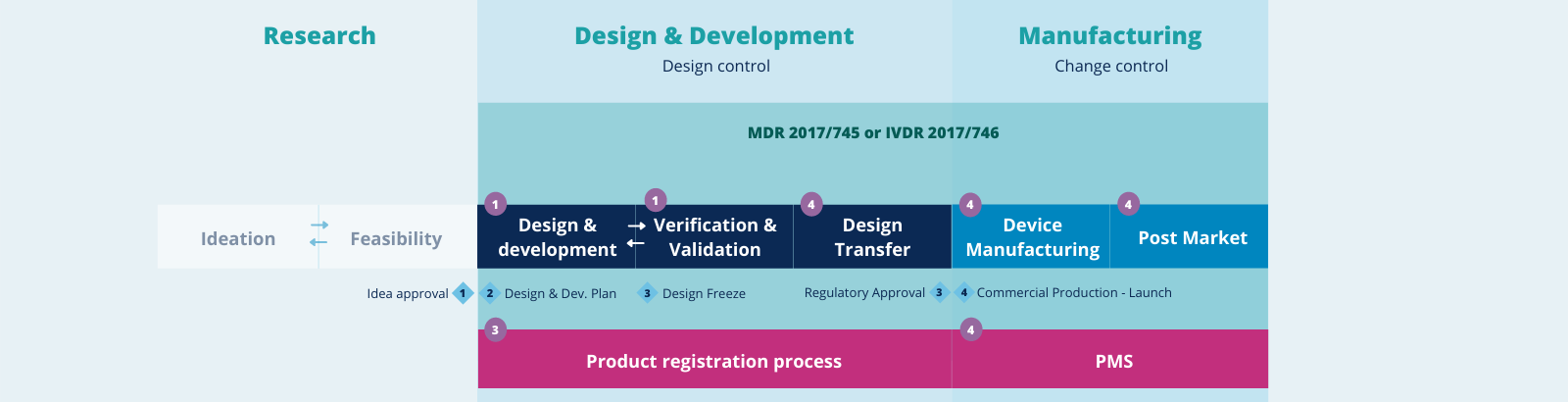
IVDs are regulated by laws that govern the safety and performance of devices across their lifetime, pre- and post-market. A risk-based classification system determines the pre- and post-market requirements for IVD.
Devices with higher risks for the patients require Notified Bodies to perform assessments on the safety and performance of the device. In this way, they ensure the continued safety and performance of IVD, pre-, and post-market.
Manufacturers must ensure to only place IVDs in the market that comply with the legislative requirements. This requires documentation of the design, development, and manufacturing process of the device including sufficient clinical evidence that shows the device is safe and performs as intended.
By establishing a quality management system and documented procedures for manufacturing and post-market monitoring activities, the quality of the product is maintained leading to continued safety and performance of the medical device during its lifecycle.

In-country Representative Services
Do you require representative services for your medical devices and/or IVDs? QbD’s Qarad is an independent partner that can act as EC-REP, CH-REP, or UKRP.
Legal Representative Clinical Trials
Are you a sponsor with offices outside the EEA and wish to conduct a clinical trial within the EEA? QbD Clinical can act as your legal representative.
Person Responsible for Regulatory Compliance (PRRC)
Are you looking for a Person Responsible for Regulatory Compliance (PRRC) for your medical devices and/or IVDs?
Technical Documentation & Clinical Evidence
Our device compliance team has the expertise to address any question or need related to clinical evidence and technical documentation of your IVD.
Why QbD Group?
Within the QbD Group, Qarad has the knowledge and expertise to support manufacturers, distributors, and importers of medical devices in complying with the legislative requirements during the complete lifecycle of a medical device.
Our clinical, regulatory, and quality experts know what needs to be done to be able to comply with the legislative requirements by which an IVD can be safely placed on the market.
Furthermore, we keep supporting businesses by managing the processes that ensure the IVD continues to be safe and effective, before and after placing it on the market.
Qarad has experience with both start-up businesses and large multinationals. We are up-to-date with the latest changes in legislation/standards and can provide project management services.
WE CAN SUPPORT YOU WITH
- Outlining the regulatory pathway of your IVD to place it on the market
- Qualification and validation of your development, testing, and manufacturing equipment and processes
- Building your risk management file
- Reviewing and building the technical file of your device
- Managing clinical performance studies and writing clinical performance study reports
- Acting as your Legal Representative for clinical performance studies
- Data management of your clinical performance studies
- Submitting your technical file to notified bodies
- Setting up, implementing, and maintaining your quality management system
- Support with ISO13485 certification
- Validation of IVD medical device software
- Performing internal audits and supplier audits
- Quality and regulatory management
- Training in ISO13485, IVDR, Risk Management, Performance Evaluation, and other IVDR-related subjects
- Acting as your EU Authorized Representative, UK Responsible Person, and Swiss Authorized Representative
- Provide a turn-key solution for eIFU
Building the business case
From concept to launch, we capture all the phases in the IVD lifecycle with the help of our extended partner network. We help you build your business case from idea to market which includes conducting revenue research, defining your business strategy and risk management, and guiding you through the entire product life cycle.
Select markets of interest (determining US and/or EU classification). Together with our dedicated partners, we predefine the minimal deliverables for your Medical Devices development strategy in our development scan, including the following phases:
- Ideation
- Market research
- Project timeline and costs
- Revenue research
- Creating a prototype
- Develop a regulatory plan
Product development phase
QbD and Qarad are aimed at helping patients benefit from new devices as quickly as possible. To ensure the quality, safety, and efficacy of MDs and IVDs, thorough Quality Management and Risk Management are crucial. We support you on the following topics:
- Design control & documentation structure
- Design verification and validation, including analytical and clinical performance testing
- Risk management
- Clinical evaluation and investigation
- Legal representative for clinical performance studies
- Design transfer to manufacturing
- Qualification and validation of development, testing and manufacturing equipment and processes
- Software validation
- Build supplier and partner network
- Develop relationships with end user
- Regulatory submission
- Implement a Quality Management System
Product launch & post-market activities
We offer full guidance for companies to bring a product to the market and support you during the commercial phase and post-market activities. Think about ensuring a robust post-market surveillance process, including performance follow-up and vigilance. Our activities include:
- Set-up distribution network
- Reimbursement strategy
- Post-market performance follow-up (PMPF
- Post-market surveillance
- Regulatory management
- Maintain Quality Management System
- External and internal audits
- Training
- Change management
- Authorized Representative in Europe
- UK Responsible Person (from Qarad UK office in London
- Swiss Authorized Representative (from From Qarad Suisse office in Lausanne)
Clients
Is your company active in one of the following businesses or related businesses? And are you in search of support in the field of Medical Devices and In Vitro Diagnostics? Let’s talk!
- Legal manufacturer
- Legal representative
- Distributor
- Contract manufacturer
- Assembler
- Third-party logistics
- Importer
Contact us
Don’t hesitate to contact us so we can listen to your needs and provide you with the right services.