
Authorized Representative Services
Your Strategic Regulatory Partner for Medical Devices & IVDs
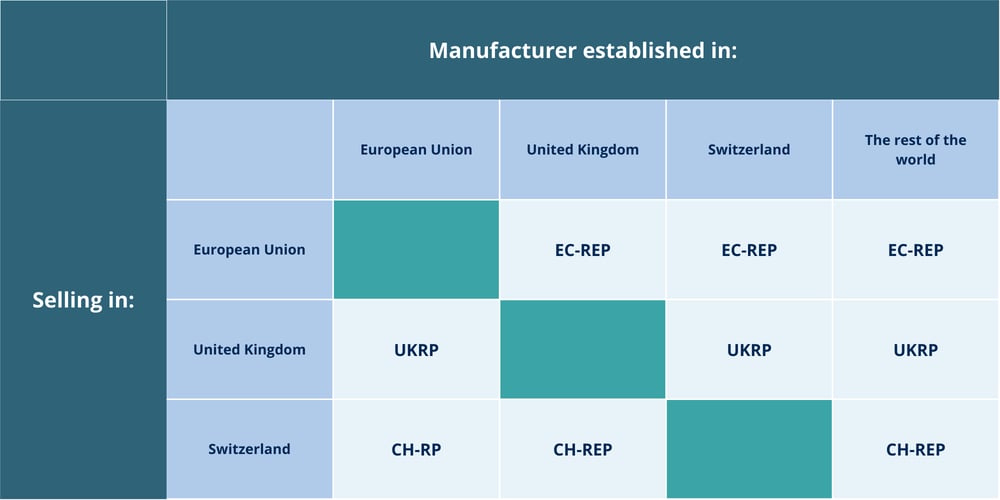
Manufacturers of medical devices and in vitro diagnostic devices (IVDs) located outside key regulatory regions are required by law to appoint an Authorized Representative (AR) in order to place products on those markets.
Whether you’re entering Europe (EU), the United Kingdom, or Switzerland, QbD Group offers experienced, independent, and fully compliant representation to help you meet all regulatory requirements.
What is an Authorized Representative?
An Authorized Representative is a legal entity established within a specific jurisdiction that acts on behalf of a non-EU / non-UK / non-Swiss manufacturer. They serve as the main point of contact between the manufacturer and local competent authorities, ensuring compliance with the region’s medical device and in vitro diagnostic device regulations and managing post-market responsibilities.
What we offer
Our regulatory experts serve as EU-REP/UKRP/CH-REP to allow easy access of IVD and MD devices to the European/UK/Swiss market.
Documentation verification
Ensure Declaration of Conformity & technical documents have been drawn up.
Compliance confirmation
Verify manufacturer has complied with registration obligations.
Labeling & Identification
Incident & Complaint Handling
Communication with Authorities
Market Surveillance Support
.jpg?width=1600&height=900&name=Legal%20Representative%20-%20Regulatory%20Affairs%20-%20QbD%20Group%20(4).jpg)
Partner with confidence: why choose QbD Group as your Authorized Representative
- Multi-Market Expertise
We ensure full compliance with EU MDR/IVDR, UK, and Swiss regulations — providing reliable representation in all three key markets. - More Than Just Representation
Beyond meeting legal requirements, we offer end-to-end support including regulatory strategy, technical documentation, and post-market surveillance. - Regulatory Expertise Accross Borders
With a vast network of regulatory consultants across the EU, UK, and Switzerland, QbD Group offers local expertise combined with cross-border regulatory consistency. - Direct, Expert Communication
You’ll work directly with experienced regulatory professionals — fast, transparent, and personalized. - Trusted Access Partner
As your EU-REP, UKRP, and CH-REP, we serve as the official point of contact for authorities and can provide the required documentation we hold on file upon request. - Authorised Representative Services for All Device Classes
We provide consistent and reliable Authorised Representative services across all device classes and IVD categories, ensuring compliance regardless of your device’s risk profile.
Trusted Authorized Representative Services for the EU, UK, and Switzerland
With over 300 customers worldwide, we are a leading provider of Authorized Representative (AR) services, helping manufacturers meet regulatory requirements in the EU, UK, and Switzerland.
Select the service that fits your needs to learn more:
Why choose QbD Group?
Global vision, local expertise
With regulatory hubs across Europe and international experience, we provide smart, scalable solutions for MedTech manufacturers.
Regulatory excellence
Backed by industry leaders like Qarad, we bring over 20 years of experience in medical device and IVD compliance.
End-to-end support
From CE marking to post-market obligations, QbD Group helps you manage the full lifecycle of your product in key markets.
Personalized, proactive approach
Our team works closely with yours to align your regulatory strategy with your business goals.
.jpg?width=1080&height=1350&name=In-country%20representative%20service%20-%20Regulatory%20Affairs%20-%20QbD%20Group%20(1).jpg)
Related content















.jpg)

Get in touch
Contact us for more information or request a free, no-obligation proposal.







.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)



.jpg)