Regulatory Affairs Updates
Discover the latest news in Regulatory Affairs
Article 5.5 IVDR enforcement on in-house devices
- Kirsten Van Garsse, Authorized Representative Director & Regulatory Affairs Manager

The key in understanding Article 5(5) is that such tests can only be run by so-called health institutions, these are facilities established within the European Union where the primary purpose is the care or treatment of patients or the promotion of public health (MDCG 2023-01) IF there is no commercially equivalent device available. For an overview of the timeline for the application of the provisions of Article 5(5), please see Figure 1.
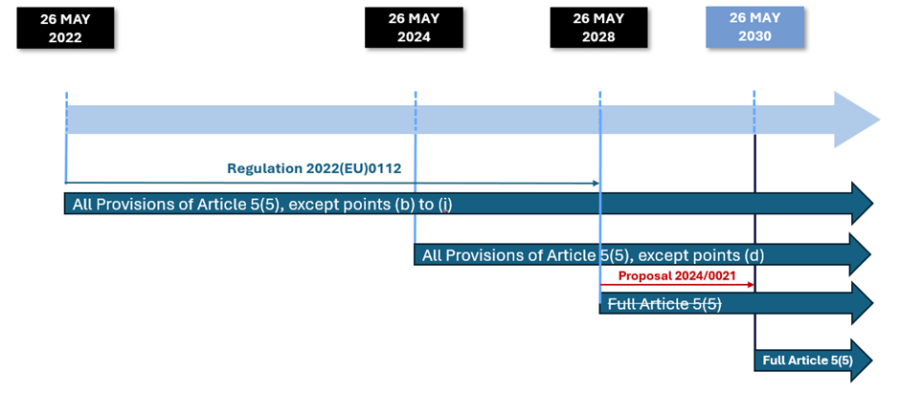
Figure 1: Timeline for the application of the different provisions of IVDR Article 5(5)
Requirements that already applied to all in-house IVDs since 26 May 2022:
- general safety and performance requirements (GSPR) set out in Annex I;
- the devices are not transferred to another legal entity;
Requirements that will enter into force as of now (26 May 2024):
- Manufacture and use of the devices occur under appropriate quality management systems;
- the health institution’s (HI’s) laboratory is compliant with EN ISO 15189 or where applicable national provisions regarding accreditation
- the health institution provides information upon request on the use of such devices to its competent authority, which shall include a justification of their manufacturing, modification and use;
- the health institution draws up a declaration which it shall make publicly available, including:
- the name and address of the manufacturing health institution,
- the details necessary to identify the devices,
- a declaration that the devices meet the general safety and performance requirements set out in Annex I to this Regulation and, where applicable, information on which requirements are not fully met with a reasoned justification therefor;
- as regards class D devices in accordance with the rules set out in Annex VIII, the health institution draws up documentation that makes it possible to have an understanding of the manufacturing facility, the manufacturing process, the design and performance data of the devices, including the intended purpose, and that is sufficiently detailed to enable the competent authority to ascertain that the general safety and performance requirements set out in Annex I to this Regulation are met. Member States may apply this provision also to class A, B or C devices in accordance with the rules set out in Annex VIII;
- the health institution takes all necessary measures to ensure that all devices are manufactured in accordance with the documentation referred to above and
- the health institution reviews experience gained from clinical use of the devices and takes all necessary corrective actions;
Ideally manufacturers have a documented procedure in place to collect clinical and performance data and to process incidents and corrective actions for IHD.
Requirements that will be enforced as of May 26th, 2028 (to be extended to 2030 under the latest proposal for an amending regulation*)
- the health institution justifies in its documentation that the target patient group’s specific needs cannot be met, or cannot be met at the appropriate level of performance by an equivalent device available on the market;
*This requirement will only be enforced by May 26th, 2030 as per the latest proposal 2024/0021 to extend the transitional provisions of the IVDR to ensure that all manufacturers have completed their transition to IVDR. It requires a comparison of the performance against IVDR claims in the Instructions for Use (IFU), but since not all manufacturers have completed their transition, this cannot be done yet.
What does this mean for you?
Article 5(5) is of importance for all Health Institutions that are involved in the development of in-house devices. If you wish to learn more on Article 5(5) and it’s relation to the new ISO 15189:2022 “Medical laboratories — Requirements for quality and competence”, please also refer to our blog.
Get in touch with our experts
Did you find this article interesting? Thanks for sharing it with your network:
Here you find all news related to Regulatory Affairs