
In Vitro Diagnostics (IVD)
The in vitro diagnostics (IVD) industry is evolving rapidly, driven by technological innovations, complex regulatory landscapes, and the increasing need for global market access.
At QbD Group, we help IVD manufacturers overcome these challenges with tailored solutions - from regulatory affairs and quality assurance to clinical evidence and technical documentation.
Partner with us to accelerate your journey from idea to patient.
Offering comprehensive IVD services
We provide end-to-end support across the entire IVD lifecycle. Our services include:

IVDD to IVDR Transition Support
We guide IVD manufacturers through the transition from IVDD to IVDR, ensuring compliance and efficiency.
- Quality Assurance: gap assessments, QMS updates, mock audits, and IVDR-compliant process development.
- Regulatory Affairs: strategic regulatory roadmaps, notified body selection, and submission support.
- Technical Documentation: clinical evidence development, risk management, usability assessments, and IVDR-compliant technical files.
.jpg?width=1600&height=900&name=Legal%20Representative%20-%20Regulatory%20Affairs%20-%20QbD%20Group%20(4).jpg)
Authorized & legal representation
Navigating regulatory approvals in the EU? We act as your trusted legal representative, ensuring compliance with stringent European standards.
- Strategic regulatory guidance
- Submission planning & execution
- Ethics committee & competent authority submissions
- Study modifications & amendments

Companion Diagnostics (CDx) support
Our expertise extends to supporting different stakeholders in the CDx ecosystem:
- Biopharma: feasibility studies, regulatory strategy, medical writing.
- IVD Manufacturers: regulatory submissions, data management, project management.
- Clinical Labs: site and supply management, quality assurance, legal representation.

Digital solutions
We offer cutting-edge software solutions to streamline regulatory compliance and quality management.
- Scilife (Smart QMS): Digital QMS for inspection readiness, risk mitigation, and compliance tracking.
- IFUcare: A full-service eIFU solution for digital technical documentation distribution.
.jpg?width=1600&height=900&name=Companion%20Diagnostics%20Services%20-%20QbD%20Group%20(2).jpg)
Outsourcing solutions
Need extra expertise for your IVD projects? We provide flexible outsourcing solutions to support your team with:
- Regulatory Affairs & Quality Assurance Specialists: experts who integrate seamlessly with your team to manage IVDR compliance.
- Clinical Research & Performance Evaluation Experts: specialists to oversee clinical and performance studies.
-
Post market surveillance (PMS) &
Post market performance follow up (PMPF) - Project Management & Medical Writing Support: ensuring smooth execution of regulatory submissions and technical documentation.
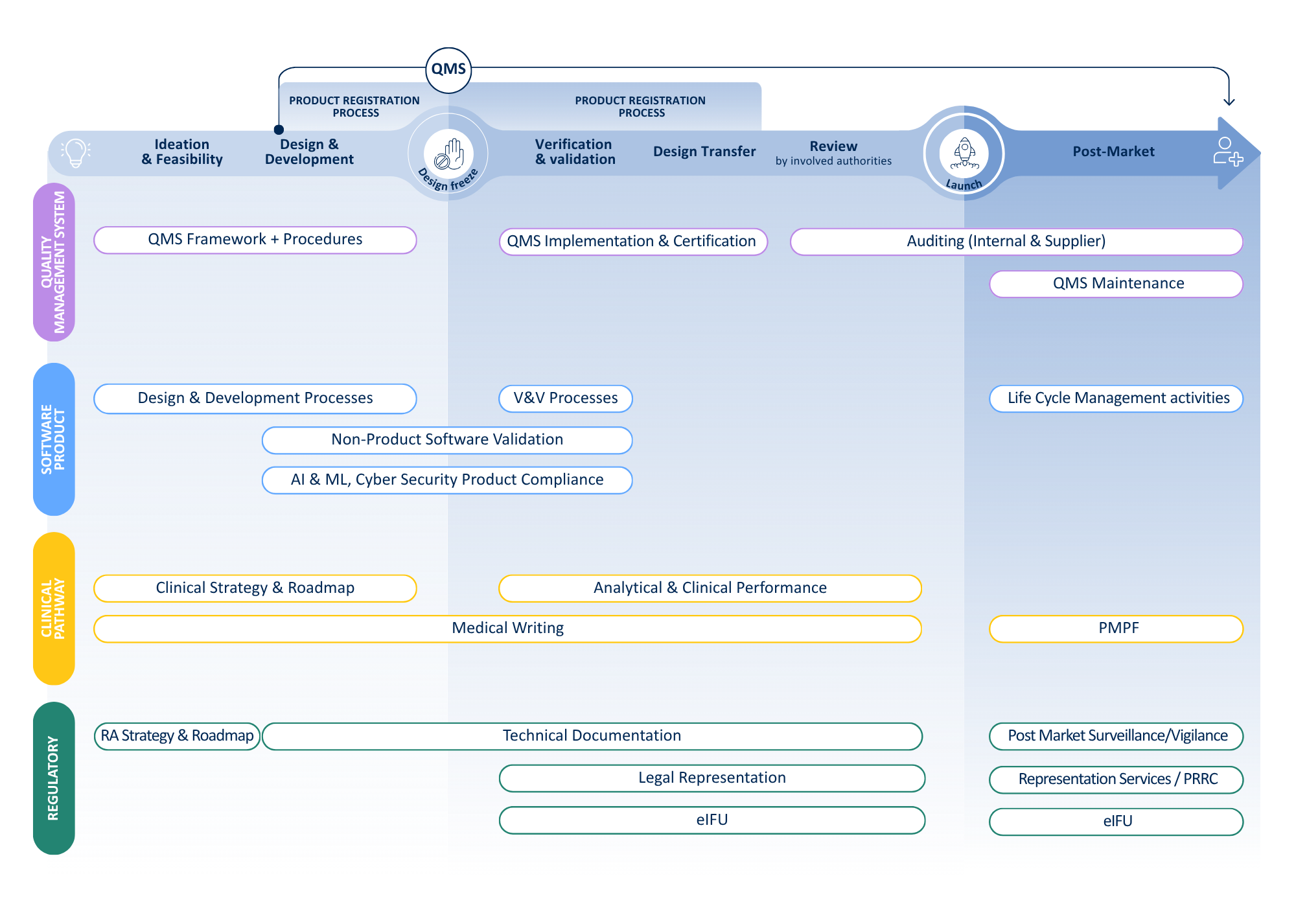
We cover the full IVD life cycle
From regulatory expertise to quality assurance and clinical evidence, we partner with you to accelerate your journey from idea to patient.
Meet our experts
Bringing an IVD product to market requires specialized knowledge in regulatory strategy, clinical performance evaluation, and quality assurance. At QbD Group, our experts combine deep industry expertise with hands-on experience to help you navigate every step of the process.
Meet some of our key team members who are ready to support your IVD journey.
Kirsten Van Garsse
20 years of experience in the IVD field
- More than 10 years in RA
- CDx, SaaMD, broad range of IVDs from Class A to Class D
- Stakeholder representative in the MDCG IVD working group and project combine
Maurizio Suppo
35+ years of experience in IVD & Medical Device Regulations
- Regulatory Affairs & Quality Systems
- European & International IVD/Medical Device Regulations
- Industry Standards & Compliance
- Strategic Leadership & Consultancy
Annelies Rotthier
15 years of experience in IVD field:
-
IVD Clinical Evidence and Medical Writing
-
IVD Product Development and IVDR Compliance
-
Strategic leadership & Consultancy
Conny Van Loon
26+ years of experience in Biomedical Devices & Product Development
- New Product Development & Market Introduction
- Project Life Cycle Management
- In Vitro Diagnostic (IVD) Regulations & Compliance
- People & Project Management
Industry challenges
Bringing an IVD product to market comes with hurdles. Here are some of the biggest challenges we help our clients navigate:
IVDR transition
Market access
Market access
Companion Diagnostics (CDx)
Companion Diagnostics (CDx)
Balancing innovation & compliance
Balancing innovation & compliance
Data integration & security
Data integration & security
Why partner with QbD IVD | Qarad?
YOUR IVD INDUSTRY EXPERT
With decades of expertise in IVD, we deliver solutions that drive success. Here’s why companies trust us:
- Unmatched Regulatory Expertise – A team with extensive IVDR certification experience and strong relationships with notified bodies.
- Proven Track Record – Over 100+ IVDR Technical Documentations completed since 2018.
- Customized, High-Quality Solutions – Strategies tailored to reduce submission timelines by 25%-50%.
- Global Reach & Industry Leadership – Supporting markets in Europe, the US, and Asia with over 650 employees and 1200+ clients worldwide.
- Full-Service CRO for IVD – With 24 years of experience, 280+ clinical performance studies conducted across 45+ analytes, and a reputation for compliance and quality excellence.
We help IVD manufacturers safely bring new devices to market, transition to IVDR, and generate the clinical evidence required for regulatory success.
Experienced in the IVD field since 1986
Full lifecycle support
Global presence
Best managed company

Get in touch with IVD experts
Get the latest IVD industry news

Related expert content
.jpg)


















.jpg)









.jpg?width=1600&height=900&name=Expert%20Regulatory%20%26%20Quality%20Support%20for%20MedTech%20Start-ups%20(1).jpg)



.png?width=800&height=800&name=Conny%20Van%20Loon%20(2).png)
.jpg)