Authorised Representative Services
Understanding the regulatory landscape for In Vitro Diagnostics (IVDs) and medical devices (MDs) in international markets can be complex.
Each country imposes unique standards, often requiring the appointment of an Authorised Representative to ensure local compliance. These complexities can create barriers to market entry and ongoing sales, demanding a precise and knowledgeable approach.
Discover how our tailored in-country representative services for IVDs and MDs can help you achieve global market success.
Unlock the doors to European, UK and Swiss markets
KEY TO SELLING YOUR DEVICE ABROAD
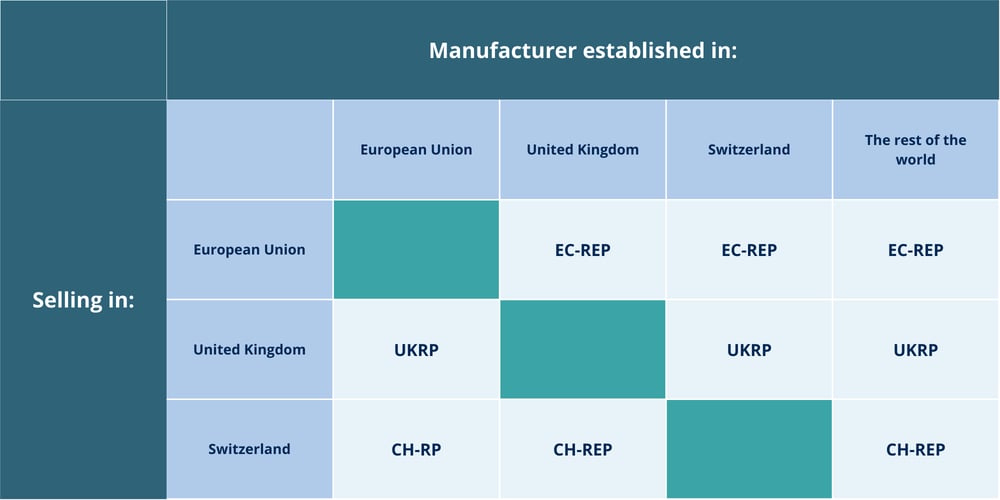
Do you require representative services to sell your Medical Devices and/or IVDs outside of your own country?
Qarad – part of the QbD Group – serves as an independent partner capable of acting as your Authorized Representative (EC-REP), Swiss Authorized Representative (CH-REP), or UK Responsible Person (UKRP).
Uncertain about the necessity of representative services for your Medical Devices and/or IVDs? Please refer to our overview below.
Why partner with QbD Group?
The Qarad experts have been active for many years as a European Authorized Representative for over one hundred different companies, and build on the regulatory and technical competence of the team. We can fulfill your need for representative services by acting as:
.jpg?width=1080&height=1350&name=In-country%20representative%20service%20-%20Regulatory%20Affairs%20-%20QbD%20Group%20(1).jpg)
Related content
%20Checklist.jpg)







Get in touch
Contact us for more information or request a free, no-obligation proposal.







.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)