.jpg?width=1600&height=900&name=Medical%20Writing%20for%20Medical%20Devices%20-%20Clinical%20-%20QbD%20Group%20(1).jpg)
Experts in Medical Device Clinical Trials
QbD Clinical is a European expert specializing in clinical trials for medical devices. We partner with companies worldwide, ensuring they meet the highest quality and safety standards, from concept to patient. We bring passion to everything we do, whether it’s a brief consultation or a full-service clinical trial.
How can we help?
We have in-depth expertise in the challenges associated with medical devices, with a focus on cardiology, vascular, neurology, and orthopedics. Unlike CROs that primarily work with pharmaceuticals, we specialize exclusively in medical device clinical trials, offering expert support tailored to your needs.
Cardiology
- Heart failure
- Acute coronary syndromes
- Atrial fibrillation
- Coronary artery disease
- Hypertension
- Others
Vascular
- Peripheral arterial disease
- Aortic aneurysms
- Carotid artery disease
- Venous thromboembolism
- Pulmonary hypertension
- Others
Neurology
- Alzheimer's disease
- Parkinson's disease
- Multiple sclerosis
- Epilepsy
- Stroke
- Others
Orthopedic
- Osteoarthritis
- Rheumatoid arthritis
- Spinal disorders
- Fracture repair
- Others

Why QbD Group?
With over 10 years of experience, QbD Clinical delivers flexible, tailored clinical solutions to help bring medical devices to market efficiently while ensuring compliance with MDR and ISO 14155.
- +52 therapeutic indications
- +250 clients worldwide
- +45 countries covered
- +650 projects delivered
- +2400 clinical sites
Always glad to share our expertise
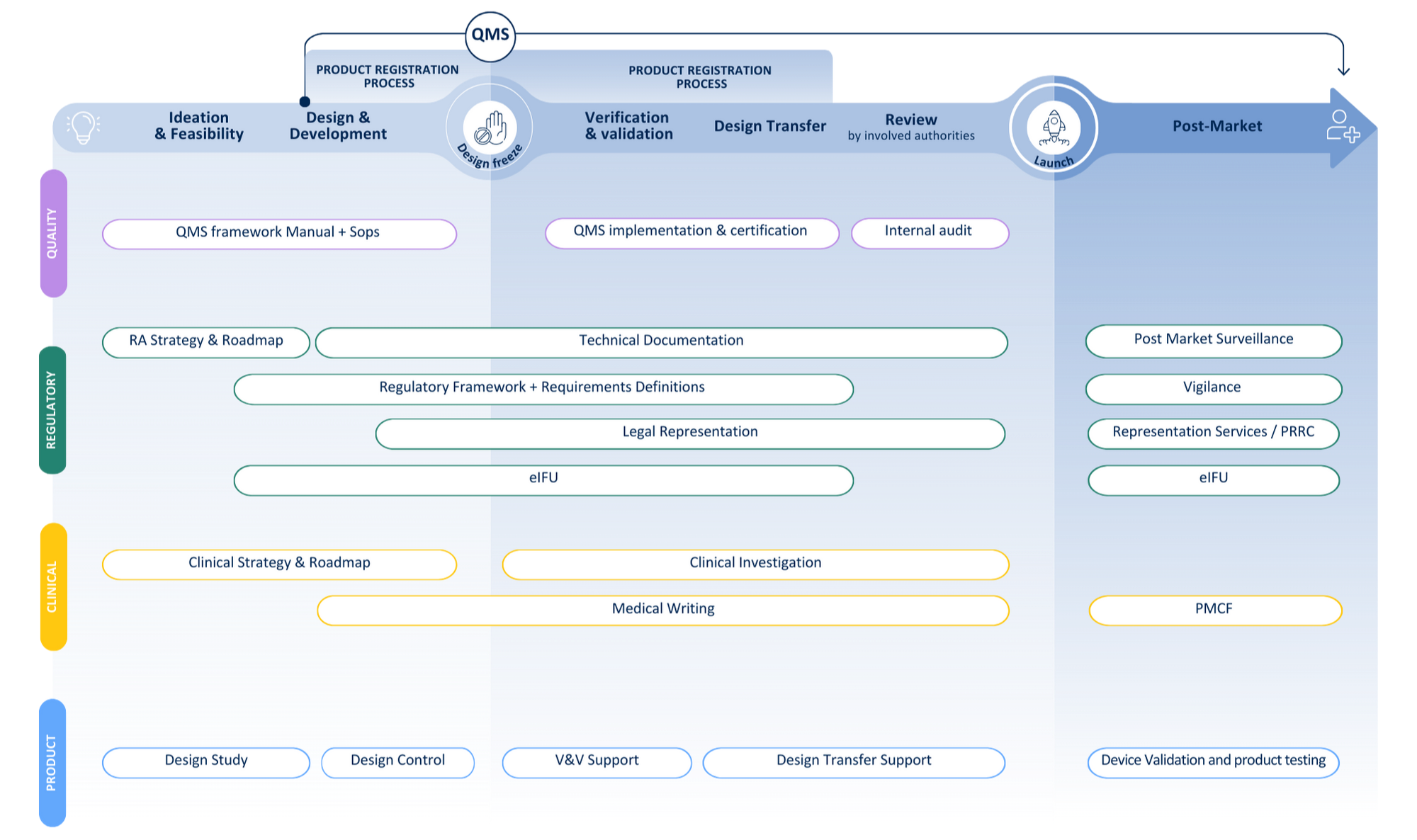
Your journey from idea to patient
Client cases: real-world results across therapeutic areas
Resources














.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)