Medical Device Software
In today’s world, medical device software manufacturers are looking to meet the unmet needs of patients and healthcare providers by leveraging the latest technological advances and taking advantage of the vast increase in digitalization.
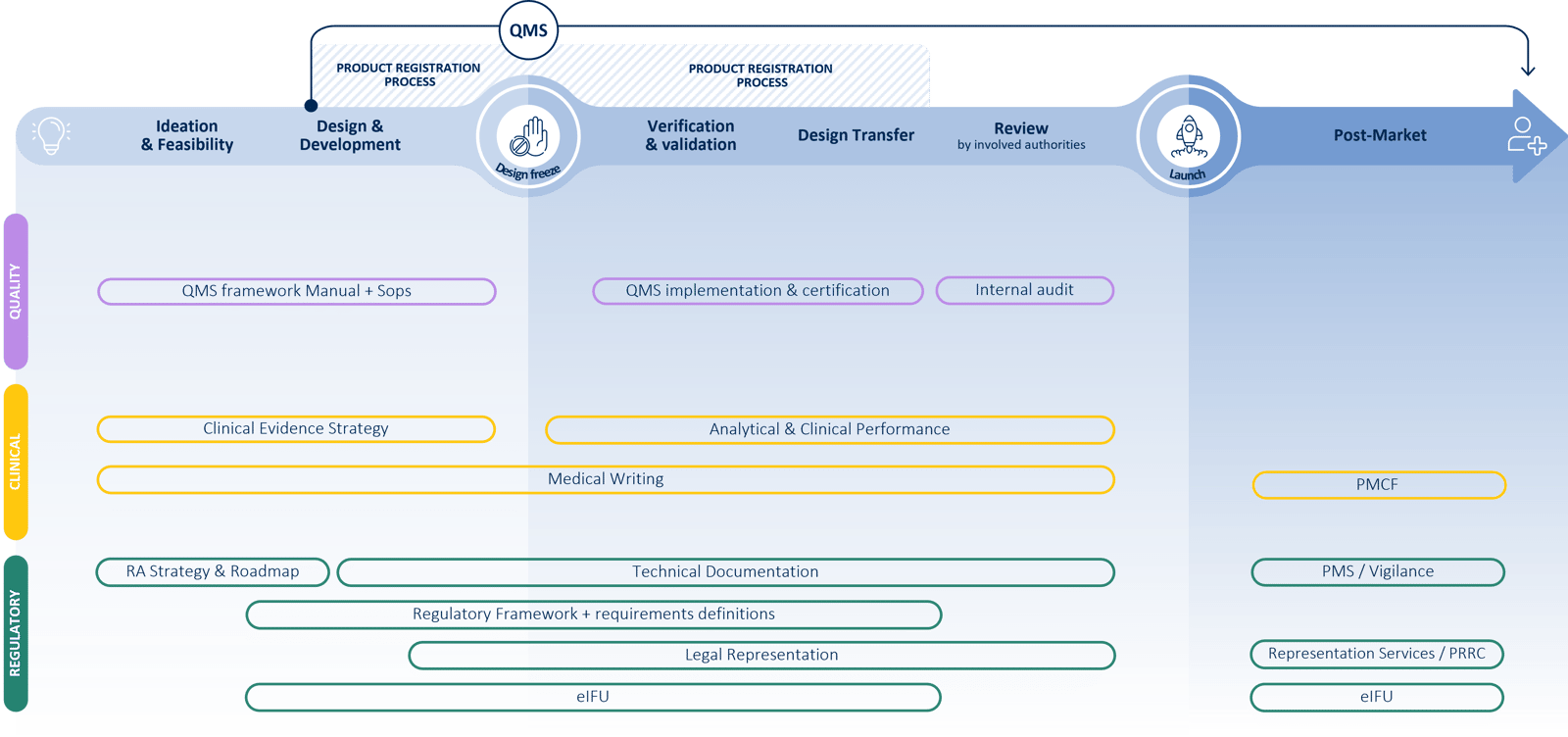
At QbD Group, we cover the full MDSW lifecycle to support you with all Regulatory, Quality, Clinical, and Design & Development challenges.
What is medical device software (MDSW)?
Software plays an important role in and around medical devices. Devices are “connected” thanks to the emergence of the IoT (Internet of Things) and its associated parts (smartphones, wearables, cloud servers, cheaper and better sensors.) Big data and AI further enhance the capabilities of medical device software. Meanwhile, the challenges of cybersecurity place another set of high demands on these innovative companies.
Medical device companies need to focus their attention on a wide range of tasks. Not only do the technical and innovative aspects of their product require their attention, but the regulatory, quality and technical aspects are challenging and sometimes underestimated.
Under the EU medical device regulations, i.e. Regulation 2017/745 (MDR), and Regulation 2017/746 (IVDR), the risk classification of medical device software (MDSW) is relatively complex and in most cases results in a higher risk class compared to the old medical device directives. This, combined with the ever-changing state of the art results in increasing regulatory, quality, and technical requirements. Medical device software manufacturers are expected to follow industry standards and guidelines applicable to MDSW (e.g., ISO13485, ISO 14971, IEC 62304, IEC 82304, IEC62366, …), as well as various regulations such as the AI Act and GDPR, while always keeping cybersecurity at the forefront (ISO 81001, …).
What is medical device software (MDSW)?
Software plays an important role in and around medical devices. Devices are “connected” thanks to the emergence of the IoT (Internet of Things) and its associated parts (smartphones, wearables, cloud servers, cheaper and better sensors.) Big data and AI further enhance the capabilities of medical device software. Meanwhile, the challenges of cybersecurity place another set of high demands on these innovative companies.
Medical device companies need to focus their attention on a wide range of tasks. Not only do the technical and innovative aspects of their product require their attention, but the regulatory, quality and technical aspects are challenging and sometimes underestimated.
Under the EU medical device regulations, i.e. Regulation 2017/745 (MDR), and Regulation 2017/746 (IVDR), the risk classification of medical device software (MDSW) is relatively complex and in most cases results in a higher risk class compared to the old medical device directives. This, combined with the ever-changing state of the art results in increasing regulatory, quality, and technical requirements. Medical device software manufacturers are expected to follow industry standards and guidelines applicable to MDSW (e.g., ISO13485, ISO 14971, IEC 62304, IEC 82304, IEC62366, …), as well as various regulations such as the AI Act and GDPR, while always keeping cybersecurity at the forefront (ISO 81001, …).
MDSW - Development, compliance, and Commercialization
Comprehensive market access strategies
Bringing medical device software to market is a multifaceted process that requires careful navigation through a rapidly changing landscape. Software plays a pivotal role in healthcare, enhancing diagnostics, personalized treatments, and patient monitoring.
To successfully commercialize, companies must understand market dynamics, including the needs of healthcare providers, payers, and patients, while aligning with regulatory requirements. Engaging key opinion leaders, demonstrating value through clinical data, and ensuring the software fits within national healthcare reimbursement systems are essential steps.
Moreover, an effective communication strategy that highlights the product’s benefits and brand message can significantly improve market entry.
Ultimately, success lies in continuous evaluation and adaptation, ensuring the software meets evolving clinical and regulatory demands.
Need some help with how to successfully bring your medical device software to market? Get in touch with us today. Our team of experts is ready to guide you through the commercialization process.
Watch our on-demand webinar to learn more about key strategies for market entry and compliance.
Medical device software development
The development of medical device software is a complex process, demanding strict adherence to international standards and regulatory requirements.
Key standards like IEC 62304, IEC 62366, and ISO 14971 govern the software development life cycle, usability, and risk management, ensuring safety and compliance throughout the process. Developers must choose between methodologies such as Waterfall or Agile, balancing the need for flexibility with the rigorous demands of medical device regulations.
Additionally, understanding software safety classifications is essential, as the required level of documentation and testing varies with the risk associated with the software. As new regulations like the AI Act emerge, alongside increasing cybersecurity demands, companies must ensure their development processes are robust, secure, and future-proof.
Successfully navigating these complexities allows organizations to deliver safe, effective software that meets both clinical and regulatory standards.
Looking to optimize your medical device software development process? Contact us for expert advice on aligning your development practices with industry standards and regulatory requirements.
Don’t forget to check out our on-demand webinar for an in-depth exploration of best practices in medical device software development.
Integrating clinical needs into MDSW design and documentation
Incorporating clinical needs into medical device software design is essential for ensuring the software’s regulatory compliance and clinical effectiveness.
This process begins with a robust clinical evaluation, as mandated by the Medical Device Regulation (MDR), requiring manufacturers to gather sufficient clinical evidence to demonstrate the software’s safety and performance. The clinical evaluation involves stages such as systematic literature reviews, which assess the current state of care and help identify potential risks and gaps. Defining the intended purpose of the device—including its medical use, target patient population, and expected clinical benefits—is critical for shaping the design.
Additionally, a comprehensive benefit-risk assessment balances the software’s positive clinical outcomes against associated risks. Post-market clinical follow-up (PMCF) ensures ongoing device safety and performance through continuous data collection and real-world evidence.
By integrating these clinical elements into both design and documentation, companies can produce software that meets regulatory demands and delivers significant value to healthcare providers and patients alike.
Ensure your software is designed with clinical needs at its core. Reach out to us for tailored support in aligning your software design with clinical requirements.
For additional insights, watch our on-demand webinar on integrating clinical strategies into medical device software development.
Technical documentation essentials for MDSW
Creating robust technical documentation (TD) is vital for compliance and market success in medical device software. The TD outlines critical information, from software qualification and classification to cybersecurity measures and product testing.
Classification follows guidelines such as the MDCG 2019-11, covering standalone software, software as an accessory, and software impacting device function. Conformity assessment requires thorough verification, validation, and cybersecurity documentation, while emerging AI standards demand transparency and risk management strategies.
Additional elements like the Unique Device Identification (UDI) system for tracking software updates and a well-chosen notified body are essential for meeting regulatory demands. Once certified, companies must maintain up-to-date documentation and conduct post-market surveillance to ensure ongoing safety and compliance.
For more information on how we can support your technical documentation needs for medical device software, reach out to us today. Our team is here to help guide you through every aspect of compliance.
You can also watch our on-demand webinar for in-depth insights and practical advice on creating and maintaining effective technical documentation.

It is undeniable that the future of medical technology and all potential applications for MDSW look exciting. However, regulations impose specific requirements throughout the entire lifecycle of a device. We’re here to help.
How we can help you
Resources

%20Checklist.jpg)





Get the latest industry news

Connect with us at these events
February
March
April
May
June
September
October
November








.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)