Nowadays, digital healthcare is taking an increasingly important place in the medical world through medical software, image processing, diagnostic support, home monitoring, etc… but all software used in a medical environment is not necessarily subject to medical regulations. Even the definition/designation of Software as a Medical Device (SaMD) can vary from country to country.
The International Medical Device Regulators Forum (IMDRF) has published guidance on Software as a Medical Device (SaMD) and what is related to it (more on that below), but it is important to realize that this guidance is not binding and thus not really followed by all countries.
In Europe, for example, the name is different: “MDSW” or “Medical Device software“. MDSW covers a different scope for software in the medical field than recommended in the IMDRF guidelines. Confused? No worries. This article will help you list the main differences between SaMD and MDSW so that you can set up a registration strategy.
SaMD: different meanings and levels of regulation
Different meanings: IMDRF as a (mere) guideline
The International Medical Device Regulators Forum (IMDRF), established in February 2011, is a forum that consists of voluntary medical device regulators from around the world who have come together to build on the strong foundational work of the Global Harmonization Task Force on Medical Devices (GHTF) and to accelerate international harmonization and convergence of medical device regulations.
Recently, the IMDRF published guidelines for “Software as a Medical Device (SaMD)” (available at imdrf.org). These guidelines are not binding. Countries that do not yet have regulations for medical software usually adopt the IMDRF’s recommendations, but a lot of countries have already adopted their own regulations.
As mentioned, the EU already defined MDSW into the MDR, for example. Consequently, most regulatory agencies have assigned a different meaning to SaMD. Often this meaning also depends on whether SaMD is built into the medical device or not.
Different levels of regulation around the world
On top of that, the level of software regulation in different countries is also quite different:
- The EU and Australia regulate a very large scope related to software;
- Most South American countries, India, China, Japan, and a few others have an average scope;
- Russia, Canada, and the US regulate software to a lesser extent than all the previous countries;
- and the rest of the world has no regulation of software at all. (See figure below)
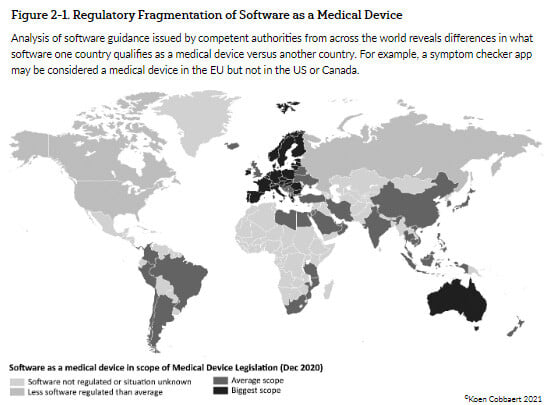
Figure 1 – Software as Medical Device- Regulatory and Market Access Implication by Koen Cobbaert & Gert Bos
SaMD versus MDSW: definitions
When it comes to software within medical environments, the terms SaMD and MDSW are often used interchangeably, but in fact, they are not.
Even if there are some similarities, they do not cover the same scope of software used in the medical field.
If you only do business in Europe, the only definition and relevant regulation you need to take care of is ‘MDSW’ regulated by the MDR, but if you’re not, it’s important to look at SaMD as well.
Below, you can find both definitions:
What is SaMD?
The IMDRF defines ‘Software as a Medical Device’ or ‘SaMD’ as
“Software intended to be used for one or more medical purposes that perform the purposes without being part of a hardware medical Device. Software is a SaMD regardless of the fact that it needs Hardware to execute. Regardless of whether it operates on general-purpose IT equipment, in “the cloud” or on the computing platform of a HW of a Medical device, as long as it is not “necessary for the HW medical device to achieve its Intended medical purpose.”
What is MDSW?
The term Medical Device Software or ‘MDSW’ is only used in the EU. (SaMD is not used in the EU). Medical Device Software for classification purposes is defined in MDR rule 11.
That rule applies to software-only devices AND hardware devices that comprise MDSW as an integral part.
The MDCG has also defined MDSW as such and provided more guidance on how to understand the MDR for software.
How are SaMD and MDSW alike and how do they differ?
As you can see, SaMD and MDSW are similar, but not synonyms. Below, we’ve listed important commonalities and differences:
What do SaMD and MDSW have in common?
- Both SaMD and MDSW fulfill one or more medical purposes independently, meaning that the software is not used to control a medical device but has its own medical purpose.
- For example, consider treatment planning that uses images from various imaging devices to calculate a treatment for a patient.
- Both apply to software operating on general-purpose computing platforms, as well as to software running on platforms that are part of a hardware medical device.
- By “general-purpose computing platform”, we mean any computer used to make the software running to analyze some X-ray images, for example, and that it is not the actual computer where the images are acquired.
- By “software running on platforms that are part of the medical device”, we mean additional software installed on the computer, used to acquire X-ray images, for example.
Are these commonalities important? They might be! If you want to register your software both in the US and in the EU, for example, it may be interesting to find out whether your software complies with both regulations (definitions).
What are the differences between SaMD and MDSW?
- MDSW also applies to software that fulfills a medical device purpose on its own but is at the same time necessary for a medical device to achieve its medical purpose (SaMD). Some examples:
- An insulin dose calculator that is also necessary to drive the infusion pump (can be an MDSW but not a SaMD).
- The software embedded in a thermometer is considered a medical device by the IMDRF and MDR, but, in the US, this does not hold.
- MDSW – due to the MDR definition of a medical device – also includes any software “supporting conception” and “alleviation of a disability”.
- MDSW does not include software that solely is intended to drive or influence the use of medical devices. E.g.:
- Software for obtaining X-ray images is used solely by the X-ray hardware, such as a mammograph or a CT scanner.
- SaMD at the international level includes software to aggregate information for medical purposes which is NOT regulated in the EU. In other words, all software treating medical information in the hospital is not considered as a medical device in Europe, but is in some other countries.
Conclusion: check all regulations
In conclusion, if you want to launch your software on the market as a medical device, it is important to check all regulations, as there are a lot of differences.
Our experts can help you