
In Vitro Diagnostics (IVD)
El sector del diagnóstico in vitro (IVD) está evolucionando rápidamente, impulsado por las innovaciones tecnológicas, la complejidad de las normativas y la creciente necesidad de acceder a los mercados mundiales.
En QbD Group, ayudamos a los fabricantes de IVD a superar estos retos con soluciones a medida, desde asuntos regulatorios y garantía de calidad hasta pruebas clínicas y documentación técnica.
Colabora con nosotros para acelerar tu camino de la idea hasta el paciente.
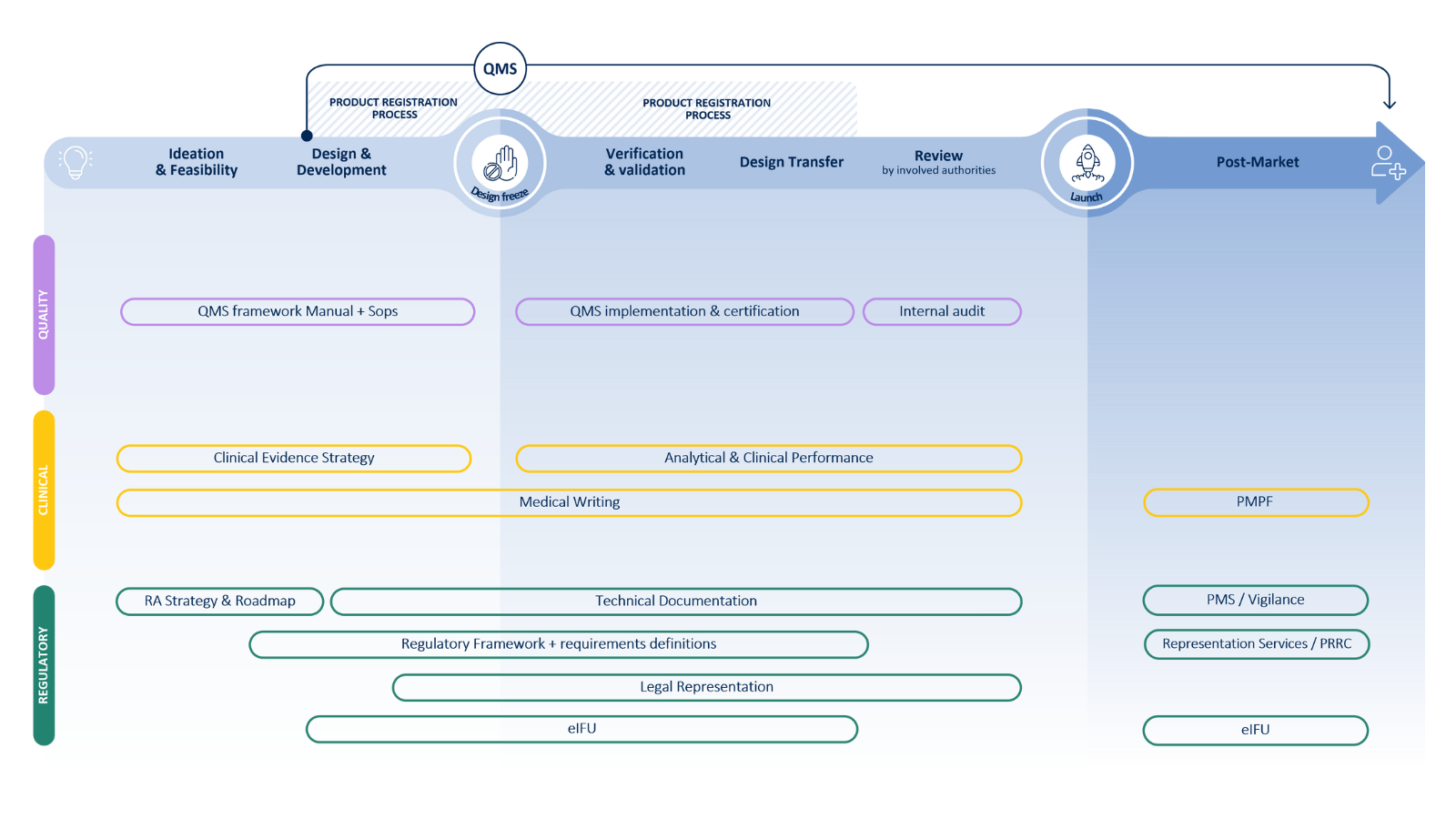
Servicios integrales de IVD
Proporcionamos asistencia integral a lo largo de todo el ciclo de vida del IVD. Nuestros servicios incluyen:

Apoyo a la transición al IVDR
Guiamos a los fabricantes de IVD a través de la transición de IVDD a IVDR, garantizando el cumplimiento y la eficiencia.
- Garantía de calidad: evaluaciones de deficiencias, actualizaciones del QMS, auditorías simuladas y desarrollo de procesos conformes con el IVDR.
- Asuntos regulatorios: hojas de ruta normativas estratégicas, selección de organismos notificados y apoyo a la presentación.
- Documentación técnica: desarrollo de pruebas clínicas, gestión de riesgos, evaluaciones de usabilidad y archivos técnicos conformes con el IVDR.
%20Support.png?width=600&height=400&name=IVD_Companion%20Diagnostics%20(CDx)%20Support.png)
Apoyo a los diagnósticos acompañantes (CDx)
Nuestra experiencia se extiende al apoyo a las diferentes partes interesadas en el ecosistema CDx:
- Biofarmacia: estudios de viabilidad, estrategia reguladora, redacción médica.
- Fabricantes de IVD: presentaciones reglamentarias, gestión de datos, gestión de proyectos.
- Laboratorios clínicos: gestión de centros y suministros, garantía de calidad, representación legal.

Servicios de IVD CRO - Estudios de evaluación del rendimiento
Realizamos estudios clínicos y de usabilidad que cumplen con:
- Reglamento DIV (UE) 2017/746
- Especificaciones comunes (UE) 2022/1107
- Requisitos de precalificación de la OMS
- ISO 20916:2019

Representación jurídica
¿Está tramitando autorizaciones reglamentarias en la UE? Actuamos como su representante legal de confianza, garantizando el cumplimiento de las estrictas normas europeas.
- Orientación normativa estratégica
- Planificación y ejecución de solicitudes
- Presentaciones a comités éticos y autoridades competentes
- Modificaciones y enmiendas de estudios

Soluciones digitales - Scilife & IFUcare
Ofrecemos soluciones de software de vanguardia para agilizar el cumplimiento de la normativa y la gestión de la calidad.
- Scilife (Smart QMS): QMS digital para la preparación de inspecciones, la mitigación de riesgos y el seguimiento de la conformidad.
- IFUcare: Una solución eIFU de servicio completo para la distribución de documentación técnica digital.

Soluciones de externalización
¿Necesita más experiencia para sus proyectos de IVD? Ofrecemos soluciones flexibles de externalización para apoyar a su equipo con:
- Especialistas en asuntos reglamentarios y garantía de calidad: expertos que se integran perfectamente con su equipo para gestionar el cumplimiento de los IVDR.
- Expertos en investigación clínica y evaluación del rendimiento: especialistas que supervisan los estudios clínicos y de rendimiento.
- Gestión de proyectos y apoyo a la redacción médica: para garantizar una ejecución fluida de las presentaciones reglamentarias y la documentación técnica.
.jpg?width=1600&height=900&name=EC-REP%20-%20Regulatory%20Affairs%20-%20QbD%20Group%20(1).jpg)
Soporte para la transición de IVDD a IVDR
QbD Group ofrece orientación experta para ayudarle a navegar la transición de manera eficiente, asegurando el cumplimiento y la preparación para el mercado en cada etapa.
Cubrimos todo el ciclo de vida del IVD
Desde la experiencia normativa hasta la garantía de calidad y las pruebas clínicas, colaboramos contigo para acelerar tu viaje de la idea al paciente.
Conoce a nuestros expertos
La introducción de un producto IVD en el mercado requiere conocimientos especializados en estrategia reguladora, evaluación del rendimiento clínico y garantía de calidad. En QbD Group, nuestros expertos combinan profundos conocimientos del sector con experiencia práctica para ayudarte en cada paso del proceso.
Conozca a algunos de los miembros clave de nuestro equipo que están preparados para ayudarte en tu camino hacia el IVD.
Kirsten Van Garsse
- Diagnóstico complementario
- Asuntos reglamentarios y sistemas de calidad
- Liderazgo estratégico y consultoría
- Desarrollo de productos DIV y cumplimiento normativo
- Documentación técnica y cumplimiento del Reglamento sobre los productos sanitarios para diagnóstico in vitro
Maurizio Suppo
Más de 35 años de experiencia en reglamentación de IVD y Medical Devices
- Regulatory Affairs y sistemas de calidad
- Normativa europea e internacional sobre IVD y dispositivos médicos.
- Normas y cumplimiento de la industria
- Liderazgo estratégico y consultoría
Annelies Rotthier
15 años de experiencia en el campo de IVD:
- Genética molecular y secuenciación de próxima generación
- Desarrollo de productos IVD y cumplimiento normativo
- Documentación técnica y cumplimiento de IVDR
- Dirección de equipos y consultoría
Conny Van Loon
Más de 26 años de experiencia en dispositivos biomédicos y desarrollo de productos
- Desarrollo de nuevos productos e introducción en el mercado
- Gestión del ciclo de vida del proyecto
- Regulaciones y cumplimiento de IVD (diagnóstico in vitro)
- Gestión de personas y proyectos
Retos del sector
Sacar un producto de IVD al mercado conlleva obstáculos. Estos son algunos de los mayores retos que ayudamos a superar a nuestros clientes:
Transición IVDR
El acceso al mercado
El acceso al mercado
Diagnóstico de compañía (CDx)
Diagnóstico de compañía (CDx)
Equilibrio entre innovación y cumplimiento
Equilibrio entre innovación y cumplimiento
Integración y seguridad de datos
Integración y seguridad de datos
¿Por qué asociarse con QbD IVD | Qarad?
TU EXPERTO EN EL SECTOR DE LA IVD
Con décadas de experiencia en IVD, ofrecemos soluciones que impulsan el éxito. He aquí por qué las empresas confían en nosotros:
- Experiencia regulatoria inigualable - Un equipo con amplia experiencia en certificación IVDR y sólidas relaciones con organismos notificados.
- Historial probado - Más de 100+ documentaciones técnicas IVDR completadas desde 2018.
- Soluciones personalizadas y de alta calidad - Estrategias adaptadas para reducir los plazos de presentación en un 25%-50%.
- Alcance global y liderazgo en la industria - Apoyo a los mercados de Europa, Estados Unidos y Asia con más de 650 empleados y más de 1200 clientes en todo el mundo.
- CRO de servicio completo para IVD - Con 24 años de experiencia, más de 250 estudios de rendimiento clínico realizados en más de 45 analitos y una reputación de excelencia en cumplimiento y calidad.
Ayudamos a los fabricantes de IVD a comercializar de forma segura nuevos dispositivos, a realizar la transición a IVDR y a generar las pruebas clínicas necesarias para el éxito regulatorio.
Más de 10 años de experiencia
Asistencia durante todo el ciclo de vida
Presencia mundial
Empresa mejor gestionada

Desde Regulatory Affairs y Quality Assurance hasta Pruebas Clínicas y Redacción Médica, nuestra amplia experiencia garantiza una solución a medida con una calidad y precisión extraordinarias".
QbD Group



Ponte en contacto con expertos en IVD
Contenido experto relacionado












.jpg)
Conéctese con nosotros en estos eventos
julio
agosto
septiembre
octubre
noviembre
Últimas noticias sobre el sector de IVD







.png?width=109&height=108&name=Pharma%20(2).png)





.png?width=800&height=800&name=Conny%20Van%20Loon%20(2).png)
.jpg)

