

Scilife™
Simplify Quality Management
Managing quality processes can feel overwhelming—staying compliant, coordinating across teams, and keeping track of CAPAs and inspections is no small task. But it doesn’t have to be this way. Scilife™ simplifies quality management, so you can regain control, reduce complexity, and focus on what matters most.
The Complete QMS Solution
Scilife™ is a user-friendly, all-in-one Quality Management Software designed to streamline every aspect of your workflow. From document control and training to CAPAs and AI-driven KPIs, Scilife ensures full traceability, integration, and compliance. Its intuitive interface makes it easy to get started, and its powerful features make staying on top of quality a breeze.
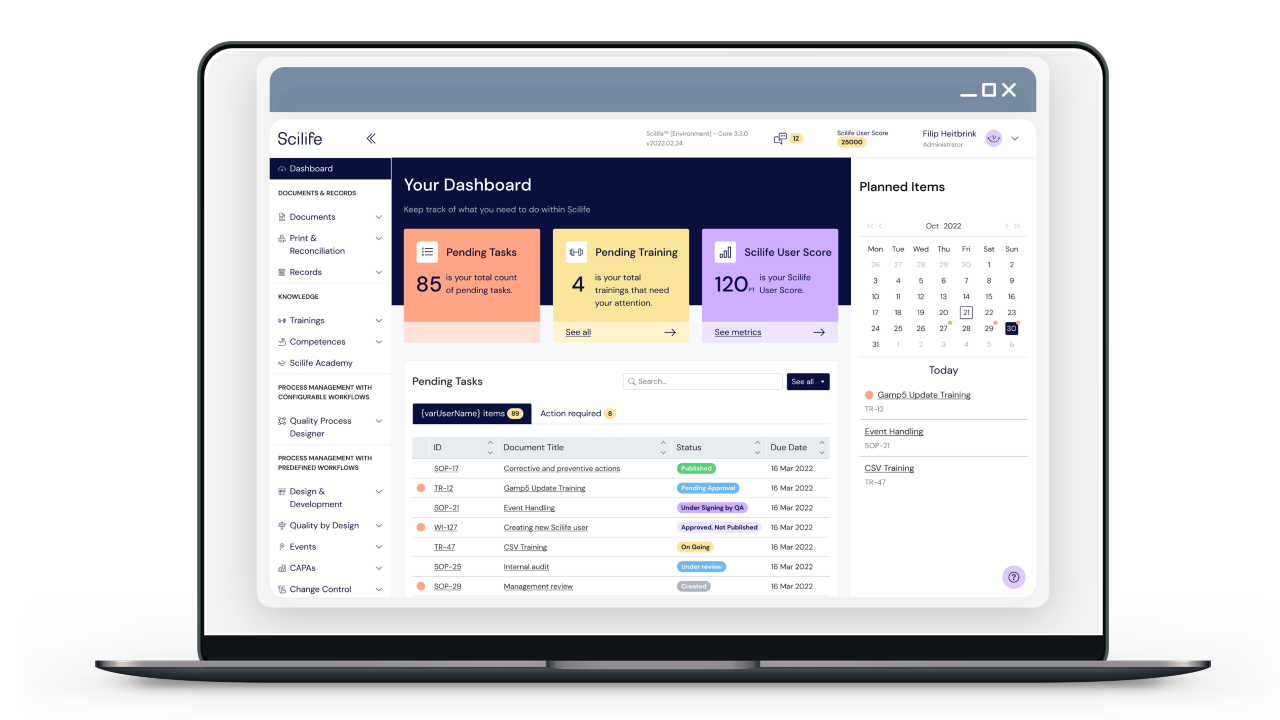
Quality management easier and cost-effective
Easy to install, easy to use
Scilife’s user-centered interface is designed to be simple, intuitive, and browser-based, ensuring that users can access Scilife from anywhere, on any device, and learn to use it quickly and easily. With a straightforward layout and clear functionality explained across four main screens, users can navigate the platform with ease and confidence.
Full Traceability, Full Control
With Scilife, you can achieve complete traceability throughout your entire quality management workflow. From raw materials to finished products, every step of the process is meticulously tracked and documented, ensuring transparency and accountability at every stage.
Always Ready for Inspection
Prepare for audits and inspections with confidence, as Scilife equips you with the tools and resources needed to maintain compliance and demonstrate adherence to industry standards. With comprehensive documentation and real-time reporting capabilities, you'll always be inspection-ready.
Smooth and Integrated QA Processes
Say goodbye to siloed systems and fragmented workflows. Scilife seamlessly integrates quality assurance processes across departments and functions, facilitating collaboration and alignment throughout the organization. Whether it's document control, change management, or risk assessment, it streamlines QA processes for maximum efficiency.
Great User Experience
The guided tour which loads automatically when new users log in for the first time, the feature explanations in the application and the field explanations in the forms, the easy-to-use design and layout, etc., all it helps users to learn easily how Scilife™ works. This ensures that users are productive soon.


How do we dot it?
Document control
Trainings
Trainings
Change control
Change control
Quality events
Quality events
CAPAs
CAPAs
KPIs (AI)
KPIs (AI)

Get in touch
Simplify your quality management with Scilife™. Whether you need assistance with document control, training management, change control, or CAPAs, our team is here to help.
Get in touch with us to learn more about how Scilife™ can transform your quality management and provide the tools you need for seamless operations.







.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)