

Companion Diagnostics (CDx)
Bringing precision medicine to patients efficiently, with fully aligned regulatory and clinical support.
What are Companion Diagnostics?
In the EU, Companion Diagnostics (CDx) are defined as devices essential for the safe and effective use of a specific medicinal product. They are designed to identify patients most likely to benefit from a treatment or to identify those at higher risk of adverse reactions.
Under IVDR, CDx are subject to enhanced regulatory scrutiny, requiring close collaboration between IVD and pharmaceutical development teams. Because CDx are ideally co-developed with pharmaceutical manufacturers, they present unique challenges that bridge two distinct regulatory pathways — an area where QbD Group offers proven expertise.
Sponsor delegation services
Pharma and diagnostic companies increasingly delegate sponsor responsibilities for IVD or CDx performance studies to trusted partners with the infrastructure, systems, and oversight required under IVDR and the Clinical Trials Regulation (CTR).
Alternative focused on IVD Sponsor Delegation only: Pharma and diagnostic companies increasingly rely on experienced partners to take responsibility for the IVD or CDx component of their clinical performance studies. By delegating defined sponsor duties to QbD Group, you gain end-to-end regulatory, quality, and clinical support under IVDR, fully aligned with CTR principles. This approach ensures compliance, visibility, and strategic alignment throughout your study, while allowing you to retain overall sponsor oversight.
QbD Group acts as your Sponsor Delegate - taking care of defined sponsor duties while ensuring full compliance, visibility, and strategic alignment.
This enables you to maintain overall sponsor control while benefiting from QbD’s cross-functional execution and compliance management.
Our Sponsor Delegation Capabilities
- Legal Representation under IVDR Article 58(4) for performance studies in the EU
- Regulatory and Clinical Strategy Development, integrating IVDR compliance and CTR-aligned study conduct
- Alliance management and support during contracting
- Study Design and Clinical Performance Study protocol development (CPSP)
- Site Management activities, including site identification and qualification
- Risk-based monitoring in accordance with ISO 20916:2024
- Competent Authority and Ethical Commission Liaison during the authorization/notification process and study conduct
- Data management strategy, collection, and analysis
- Medical Writing and Submission Package Development (protocols, IBs, Informed Consent forms, country-specific documents)
- Safety Management and Reporting in line with IVDR and local requirements
- Sponsor Oversight and Training for sponsor teams on QA, RA, and clinical-related topics
This delegated model is ideal for biopharma and diagnostic sponsors performing biomarker or CDx performance studies in the EU or UK that require an experienced partner to handle end-to-end sponsor activities.

Sponsor delegation services
Pharma and diagnostic companies increasingly delegate sponsor responsibilities for IVD or CDx performance studies to trusted partners with the infrastructure, systems, and oversight required under IVDR and the Clinical Trials Regulation (CTR).
Alternative focused on IVD Sponsor Delegation only: Pharma and diagnostic companies increasingly rely on experienced partners to take responsibility for the IVD or CDx component of their clinical performance studies. By delegating defined sponsor duties to QbD Group, you gain end-to-end regulatory, quality, and clinical support under IVDR, fully aligned with CTR principles. This approach ensures compliance, visibility, and strategic alignment throughout your study, while allowing you to retain overall sponsor oversight.
QbD Group acts as your Sponsor Delegate - taking care of defined sponsor duties while ensuring full compliance, visibility, and strategic alignment.
This enables you to maintain overall sponsor control while benefiting from QbD’s cross-functional execution and compliance management.
Our Sponsor Delegation Capabilities
- Legal Representation under IVDR Article 58(4) for performance studies in the EU
- Regulatory and Clinical Strategy Development, integrating IVDR compliance and CTR-aligned study conduct
- Alliance management and support during contracting
- Study Design and Clinical Performance Study protocol development (CPSP)
- Site Management activities, including site identification and qualification
- Risk-based monitoring in accordance with ISO 20916:2024
- Competent Authority and Ethical Commission Liaison during the authorization/notification process and study conduct
- Data management strategy, collection, and analysis
- Medical Writing and Submission Package Development (protocols, IBs, Informed Consent forms, country-specific documents)
- Safety Management and Reporting in line with IVDR and local requirements
- Sponsor Oversight and Training for sponsor teams on QA, RA, and clinical-related topics
This delegated model is ideal for biopharma and diagnostic sponsors performing biomarker or CDx performance studies in the EU or UK that require an experienced partner to handle end-to-end sponsor activities.
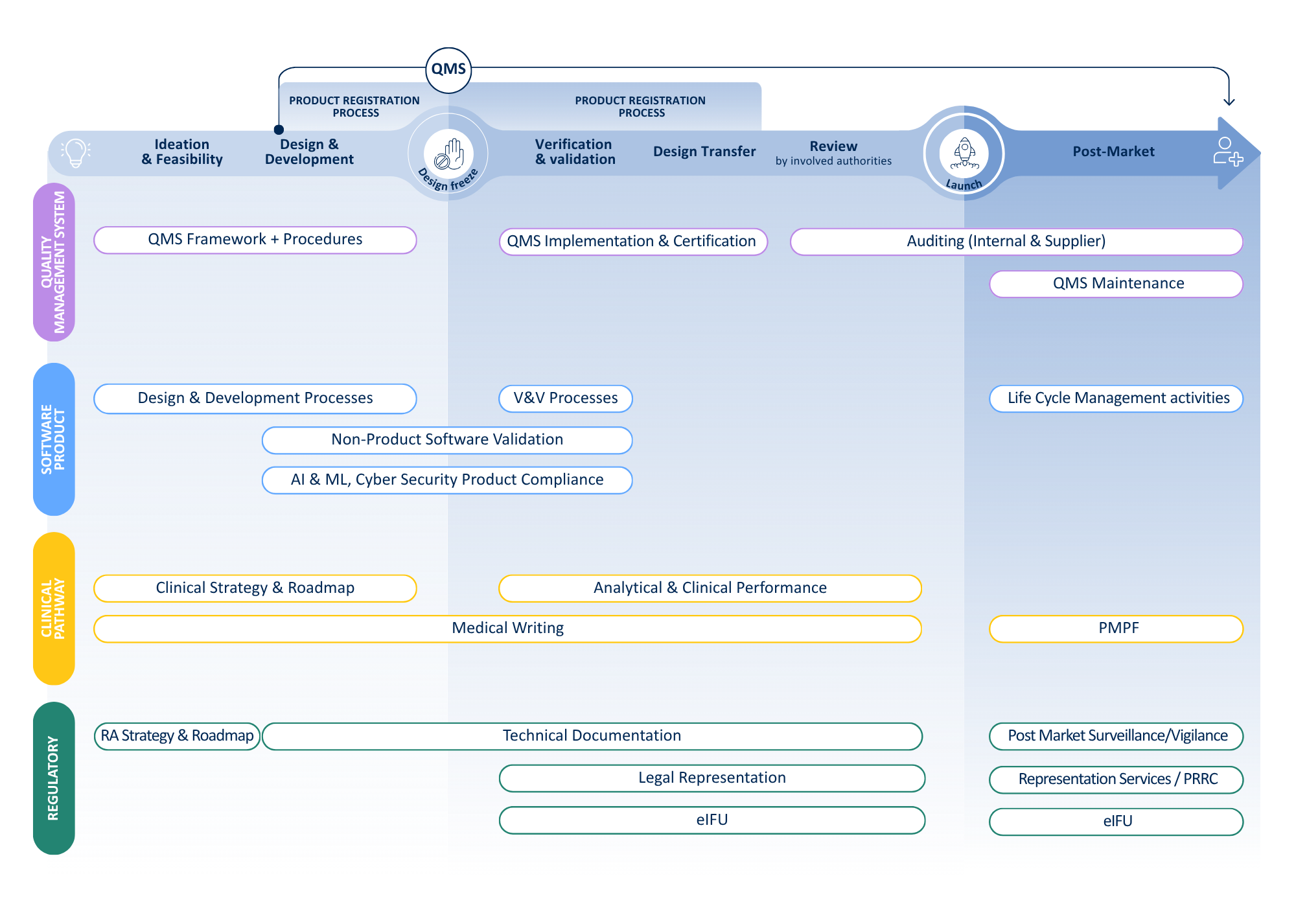
Our companion diagnostics services
Device support
Medicinal product support

Device support
Our services in device development encompass strategic guidance, project management, and comprehensive operational support throughout all phases of CDx development:
- Notified Body engagement: Support with selection, contracting, and ongoing liaison.
- Submission and review support: Guidance through the submission and review process with the Notified Body.
- Quality Management System implementation: Assistance in setting up or optimizing a compliant QMS.
- Clinical evidence strategy: Define an optimal, compliant, and pragmatic approach to demonstrate scientific validity, analytical performance, and clinical performance.
- Clinical performance study design & implementation: Design and full-service execution of Annex XIV-compliant performance studies in the EU.
- Legal representation: Acting as your legal representative for study applications under IVDR Article 58(4).
- Medical writing & submission package: Preparation or review of study documentation, with tailored submission strategies for each EU member state.
- Risk management: support in developing a comprehensive risk management file in line with ISO 14971.
- Representative services: Acting as your EU Authorized Representative, UK Responsible Person (UKRP), and Swiss Authorised Representative
- IVDR & CDx Training: On-site or remote training on regulatory and operational aspects of CDx studies across the EU, UK, and Switzerland.

Medicinal product support
At QbD Group, we understand that the successful development of a medicinal product that relies on a companion diagnostic (CDx) requires a coordinated and well-structured development plan. The interdependence between the drug and the diagnostic impacts key regulatory, clinical, and quality aspects throughout development — from proof of concept to marketing authorisation.
Building on the companion diagnostic aspects, at QbD Group, we can also support the development of the medicinal product, ensuring that all related requirements are seamlessly integrated into the regulatory and development strategy.
Services provided by QbD Group in this context include, but are not limited to:
- Regulatory Strategy and Pathway Definition: identifying the most appropriate regulatory route for the medicinal product in view of its dependence on a companion diagnostic; alignment with EMA expectations for co-development.
- Target Product Profile (TPP) and Biomarker Integration: defining the therapeutic indication, patient population, and diagnostic use conditions within the TPP and clinical development plan.
- Clinical Development Planning and Study Design: ensuring the inclusion of diagnostic-related endpoints, stratification criteria, and patient selection strategy within clinical trials supporting the medicinal product.
- Clinical Trial Applications (CTAs): preparation of the required documentation (e.g., IMPD, IB, protocol, etc) and submission to the relevant health authorities and ethics committees, including full support during the validation and assessment phases of the application.
- MAA Dossier Preparation: drafting and integrating relevant sections of the CTD for the medicinal product, reflecting the link with the diagnostic and demonstrating how CDx performance supports the clinical benefit–risk profile.
- Interactions with Diagnostic Partners and Health Authorities: assisting with coordination between the drug sponsor and the diagnostic developer; preparation for EMA scientific advice or parallel consultations to ensure consistent messaging and alignment of development timelines.
- Quality Management System implementation: Development and audit-readiness for compliant QMS integration.
- Post-Approval Support: managing updates to the medicinal product dossier related to diagnostic changes, new biomarkers, or expanded indications.

Trusted partner for seamless CDx and IVDR compliance.
Russell Henderson


Why partner with QbD Group?
We bring an integrated perspective: our team understands not only the regulatory expectations but also how the medicinal product’s development, evidence generation, and documentation must evolve alongside its companion diagnostic. This understanding ensures that the medicinal product dossier remains robust, compliant, and strategically aligned with market access objectives.
- Unparalleled cross-functional expertise: Our teams combine IVD, pharmaceutical, and clinical operations knowledge, enabling seamless coordination across dual regulatory frameworks.
- Trusted Sponsor Delegation partner: We act as a true Sponsor Delegate for the IVD/CDx component, managing regulatory, clinical, and QMS obligations with full transparency and client oversight.
- End-to-end CDx lifecycle support: From early feasibility to post-market evidence generation, we provide full-service coverage through our integrated Regulatory, Quality, and Clinical teams.
- Strategic insights for compliance and speed: Our experts design optimized development pathways that anticipate regulatory expectations and accelerate IVDR-compliant study execution.
- Global reach with local precision: QbD Group operates across the EU, UK, and CH, combining central coordination with regional compliance, site management, and monitoring expertise.
.jpg?width=1600&height=900&name=Companion%20Diagnostics%20Services%20-%20QbD%20Group%20(2).jpg)

Get in touch
Whether you are developing a first-in-human CDx study, managing an IVDR transition, or seeking a sponsor delegate for the IVD/CDx component of your study, QbD Group provides regulatory assurance, operational excellence, and strategic foresight to bring your diagnostic innovation to patients safely and efficiently.
Contact our team to discuss your study design, sponsor delegation needs, or regulatory challenges.
Related expert content


















.jpg)
Get the latest news








.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)
.jpg)