

Experts in IVD Clinical Performance Studies
Ensuring comprehensive clinical evidence and demonstrating clinical performance is a challenging process. Notified Bodies often highlight that initial submission packages are incomplete, with over 40% of deficiencies relating to clinical evidence.
Are you looking for an experienced partner to generate trustworthy and accurate clinical performance for your IVD device, ensuring full regulatory compliance?
As a leading full-service Contract Research Organization (CRO), we can help you overcome the complexities that come with clinical performance studies and ensure they are conducted with the highest level of expertise and quality.
What are IVD clinical performance studies?
IVD clinical performance studies are essential in assessing and confirming the safety and performance of In Vitro Diagnostic Medical Devices (IVD). The IVD Regulation (EU) 2017/746, ISO20916:2024, and the World Health Organization (WHO) have defined stringent requirements that manufacturers must comply with.
We offer clinical performance studies in collaboration with renowned clinical laboratories, blood banks, and state-of-the-art private laboratories across Europe. As a sponsor, you can be confident that we will handle every aspect of the clinical performance study for your IVD, leaving you free to focus on your core business activities.
.jpg?width=1080&height=1350&name=portrait%20image%20-%20website%20(3).jpg)
Why are these studies important?
- Clinical Performance Studies are not just a regulatory requirement – they’re critical for proving that your IVD device performs reliably and safely under real-world conditions.
- These studies help safeguard the health and safety of patients and users, while also ensuring that your device meets the necessary criteria for market approval.
- With evolving regulations and growing industry standards, having accurate clinical evidence is essential to gaining regulatory approval and ensuring market success for your IVD devices.
How can we help?
We are a CRO specialized in In Vitro Diagnostics (IVD), with in-depth expertise in the unique challenges of IVD performance studies. Our focus areas include infectious diseases, oncology, and endocrinology. Unlike CROs that treat IVD support as an add-on to their medical device services, we deliver dedicated expertise and streamlined support across the entire IVD lifecycle.
Study strategy
Site selection
Site selection
Protocol development
Protocol development
Regulatory support
Regulatory support
Training & monitoring
Training & monitoring
Data analysis & reporting
Data analysis & reporting
Our expertise
At QbD Group, we bring deep therapeutic expertise to every clinical project. Our Clinical Solutions team is particularly specialized in supporting studies within three key areas: infectious diseases, oncological conditions, and endocrinology. With hands-on experience and a solid understanding of regulatory and clinical complexities, we help our partners navigate these high-impact therapeutic fields with confidence and precision.
Infectious diseases
- Covid-19
- HIV
- HCV
- Syphilis
- Influenza
- Malaria
Oncological conditions
- Bladder cancer
- Breast cancer
- Lymphoma
- Liver cancer (Hepatocellular Carcinoma, HCC)
- Colorectal cancer (Metastatic CRC. mCRC)
- Pancreatic cancer
- Non-Small Cell Lung Cancer (NSCLC)
Endocrinology
- Diabetes Mellitus
- Hypercholesterolemia
- NASH
- Esophagitis

Probably the best place to go in Europe for IVD consulting needs and clinical services.
GNA Biosolutions GmbH

Why QbD IVD | Qarad?
From initial study design to final closure, we offer a complete spectrum of CRO services. Here’s why manufacturers trust us globally:
- Regulatory compliance: We ensure your studies fully comply with IVDR, Common Specifications, ISO 20916:2019, WHO-PQ Technical Specification Series, and national or regional requirements for Ethics Committee approval.
- Experienced network: Benefit from our strong network of renowned clinical laboratories, blood banks, and private laboratories across Europe.
- Tailored solutions: We offer customized services designed to meet the specific needs of your IVD device.
- Diverse environments: We excel in various intended user environments, including clinical labs, near-patient test settings, and self-test studies.
- Proven track record: With over 300 studies completed, we bring unparalleled experience in infectious disease diagnostics and compliance with IVDR and WHO requirements.
- Notified Bodies recognition: We offer guaranteed quality esteemed by Notified Bodies
- Certified excellence: We hold ISO 13485 & ISO 27001 certifications, ensuring the highest standards of quality and security.
.jpg?width=1600&height=900&name=IVD%20Clinical%20Performance%20Studies%20-%20Clinical%20-%20QbD%20Group%20(1).jpg)
Why QbD IVD | Qarad?
From initial study design to final closure, we offer a complete spectrum of CRO services. Here’s why manufacturers trust us globally:
- Regulatory compliance: We ensure your studies fully comply with IVDR, Common Specifications, ISO 20916:2019, WHO-PQ Technical Specification Series, and national or regional requirements for Ethics Committee approval.
- Experienced network: Benefit from our strong network of renowned clinical laboratories, blood banks, and private laboratories across Europe.
- Tailored solutions: We offer customized services designed to meet the specific needs of your IVD device.
- Diverse environments: We excel in various intended user environments, including clinical labs, near-patient test settings, and self-test studies.
- Proven track record: With over 300 studies completed, we bring unparalleled experience in infectious disease diagnostics and compliance with IVDR and WHO requirements.
- Notified Bodies recognition: We offer guaranteed quality esteemed by Notified Bodies
- Certified excellence: We hold ISO 13485 & ISO 27001 certifications, ensuring the highest standards of quality and security.
What our clients say about us
At QbD Clinical, client feedback isn’t just a checkbox, it’s part of our DNA.
We’re proud to share our 2024 client satisfaction scores, reflecting the trust and collaboration we build every day.
4.5/5 average client rating
4.5/5 average client rating
288 clinical studies
288 clinical studies
100+ happy clients
100+ happy clients
4.5/5 – Recommended by our clients
4.5/5 – Recommended by our clients
4.8/5 – Expertise across clinical domains
4.8/5 – Expertise across clinical domains
4.6/5 – High willingness to reuse our services
4.6/5 – High willingness to reuse our services
4.6/5 – Clear communication & responsiveness
4.6/5 – Clear communication & responsiveness
4.6/5 – Consistently meeting quality expectations
4.6/5 – Consistently meeting quality expectations
Always glad to share our expertise
Your journey from idea to patient
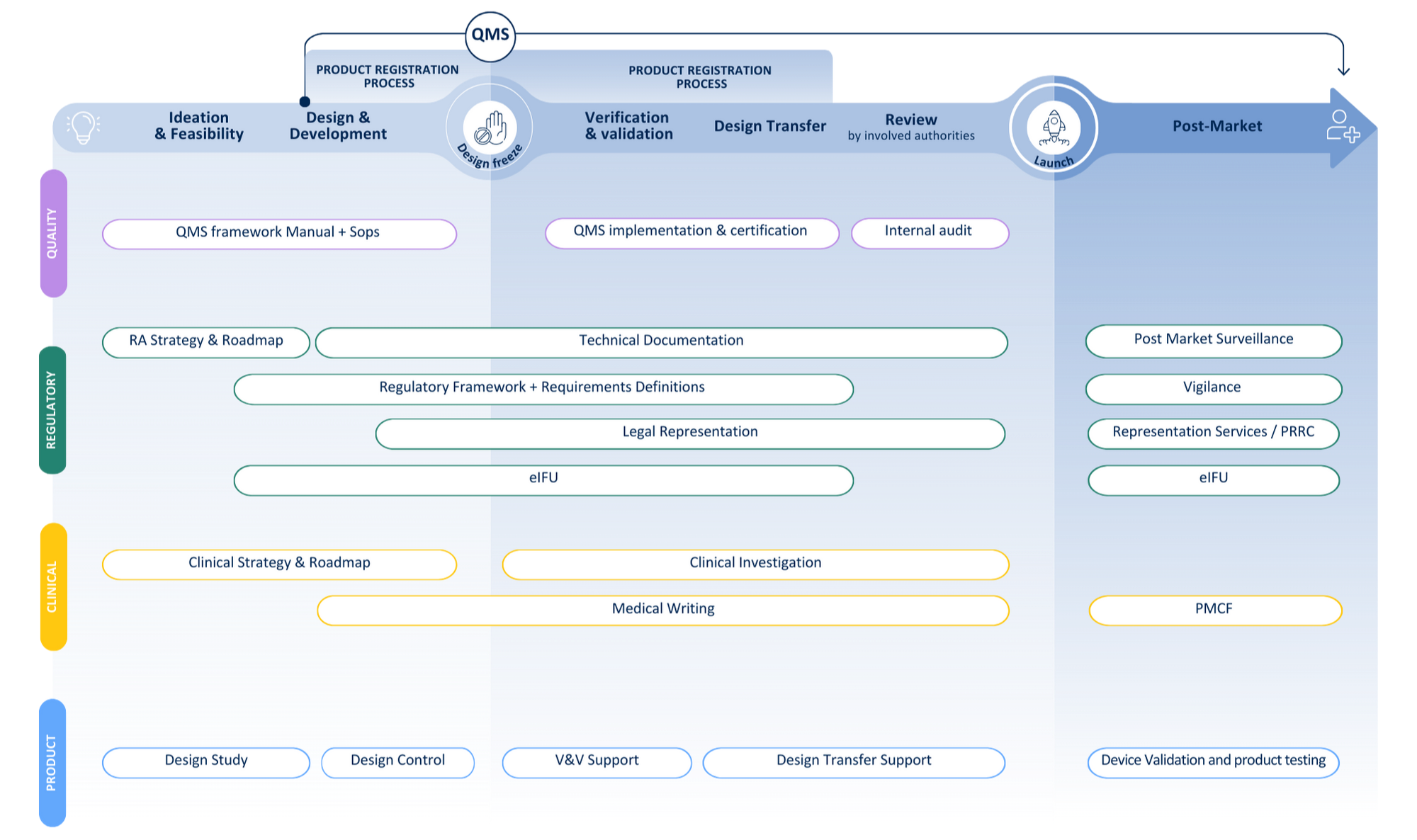
Recent client cases: combining compliance & clinical expertise
Explore how we helped two IVD manufacturers navigate complex study requirements and compliance frameworks — from IVDR and WHO alignment to full study sponsorship.

Combination of IVDR & WHO requirements
-
Helped bring a Class D product for near-patient and self-testing to the EU market and achieve WHO-PQ approval.
-
Combined IVDR and WHO requirements to reduce sample count and streamline processes.
-
Set up 4 distinct studies:
-
Clinical study (IVDR + WHO)
-
Near-patient test (IVDR)
-
Self-test (IVDR)
-
Self-test (WHO)
-
-
Acted as legal representative for EC/CA approvals.
Result: First-time-right compliance, fewer samples, and ongoing collaboration on 4 additional products.

Study Sponsorship Support
-
Took over IVDR performance study sponsorship for a U.S.-based IVD manufacturer.
-
Managed 8,000 samples across 100+ sites in 10 EU countries.
-
Provided support for study design, medical writing, GMS & GDPR compliance.
-
Coordinated EC/CA documentation and full Dx site monitoring.
-
Acted as legal representative throughout the study lifecycle.
Result: Streamlined execution, expert guidance, and long-term planning flexibility.

Get in touch
As a sponsor, you can be confident that we will handle every aspect of the clinical performance study for your IVD, leaving you free to focus on your core business activities.

Related resources








.jpg)










.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)
