
Experts in IVD Clinical Performance Studies
Ensuring comprehensive clinical evidence and demonstrating clinical performance is a challenging process. Notified Bodies often highlight that initial submission packages are incomplete, with over 40% of deficiencies relating to clinical evidence.
Are you looking for an experienced partner to generate trustworthy and accurate clinical performance for your IVD device, ensuring full regulatory compliance?
As a leading full-service Contract Research Organization (CRO) who has supported 300+ studies for 50+ clients, we can help you overcome the complexities that come with clinical performance studies and ensure they are conducted with the highest level of expertise and quality.
How can we help?
We are a CRO specialized in In Vitro Diagnostics (IVD), with in-depth expertise in the unique challenges of IVD performance studies. Our focus areas include infectious diseases, oncology, and endocrinology. Unlike CROs that treat IVD support as an add-on to their medical device services, we deliver dedicated expertise and streamlined support across the entire IVD lifecycle. We are a flexible business partner, supporting both full-service outsourcing, as well as one-off solutions.
Study strategy
Site selection
Site selection
Protocol development
Protocol development
Regulatory support
Regulatory support
Training & monitoring
Training & monitoring
Data analysis & reporting
Data analysis & reporting
Our expertise
At QbD Group, we bring deep therapeutic expertise to every clinical project. Our QbD IVD Qarad team is particularly specialized in supporting studies within three key areas: infectious diseases, oncological conditions, and endocrinology. With hands-on experience and a solid understanding of regulatory and clinical complexities, we help our partners navigate these high-impact therapeutic fields with confidence and precision.
Infectious diseases
- Serology (HIV, HBV, HCV, Syphilis)
- Respiratory diseases (Covid-19, Influenza A/B, RSV)
- STIs
- Tropical diseases (Malaria, Dengue)
- Gastrointestinal (C. difficile, H. Pylori)
Oncological conditions
- Bladder cancer
- Breast cancer
- Lymphoma
- Liver cancer (Hepatocellular Carcinoma, HCC)
- Colorectal cancer (Metastatic CRC. mCRC)
- Pancreatic cancer
- Non-Small Cell Lung Cancer (NSCLC)
Endocrinology
- Fertility (hCG, LH)
- Diabetes (Glucose, HbA1C)
Other
- Blood grouping
- Hemoglobin
- Troponin I
- Lipoprotein (a)

Probably the best place to go in Europe for IVD consulting needs and clinical services.
GNA Biosolutions GmbH

Why QbD IVD | Qarad?
From initial study design to final closure, we offer a complete spectrum of CRO services. Here’s why manufacturers trust us globally:
- Regulatory compliance: We ensure your studies fully comply with IVDR, Common Specifications, ISO 20916:2019, WHO-PQ Technical Specification Series, and national or regional requirements for Ethics Committee approval.
- Experienced network: Benefit from our strong network of renowned clinical laboratories, blood banks, and private laboratories across Europe.
- Tailored solutions: We offer customized services designed to meet the specific needs of your IVD device.
- Diverse environments: We excel in various intended user environments, including clinical labs, near-patient test settings, and self-test studies.
- Proven track record: With over 300 studies completed, we bring unparalleled experience in infectious disease diagnostics and compliance with IVDR and WHO requirements.
- Notified Bodies recognition: We offer guaranteed quality esteemed by Notified Bodies
- Certified excellence: We hold ISO 13485 & ISO 27001 certifications, ensuring the highest standards of quality and security.
.jpg?width=1600&height=900&name=IVD%20Clinical%20Performance%20Studies%20-%20Clinical%20-%20QbD%20Group%20(1).jpg)
Why QbD IVD | Qarad?
From initial study design to final closure, we offer a complete spectrum of CRO services. Here’s why manufacturers trust us globally:
- Regulatory compliance: We ensure your studies fully comply with IVDR, Common Specifications, ISO 20916:2019, WHO-PQ Technical Specification Series, and national or regional requirements for Ethics Committee approval.
- Experienced network: Benefit from our strong network of renowned clinical laboratories, blood banks, and private laboratories across Europe.
- Tailored solutions: We offer customized services designed to meet the specific needs of your IVD device.
- Diverse environments: We excel in various intended user environments, including clinical labs, near-patient test settings, and self-test studies.
- Proven track record: With over 300 studies completed, we bring unparalleled experience in infectious disease diagnostics and compliance with IVDR and WHO requirements.
- Notified Bodies recognition: We offer guaranteed quality esteemed by Notified Bodies
- Certified excellence: We hold ISO 13485 & ISO 27001 certifications, ensuring the highest standards of quality and security.
What our clients say about us
At QbD IVD Qarad, client feedback isn’t just a checkbox, it’s part of our DNA.
We’re proud to share our 2024 client satisfaction scores, reflecting the trust and collaboration we build every day.
288 clinical studies
288 clinical studies
100+ happy clients
100+ happy clients
4.5/5 average client rating
4.5/5 average client rating
4.5/5 – Recommended by our clients
4.5/5 – Recommended by our clients
4.8/5 – Expertise across clinical domains
4.8/5 – Expertise across clinical domains
4.6/5 – High willingness to reuse our services
4.6/5 – High willingness to reuse our services
4.6/5 – Clear communication & responsiveness
4.6/5 – Clear communication & responsiveness
4.6/5 – Consistently meeting quality expectations
4.6/5 – Consistently meeting quality expectations
Always glad to share our expertise
Your journey from idea to patient
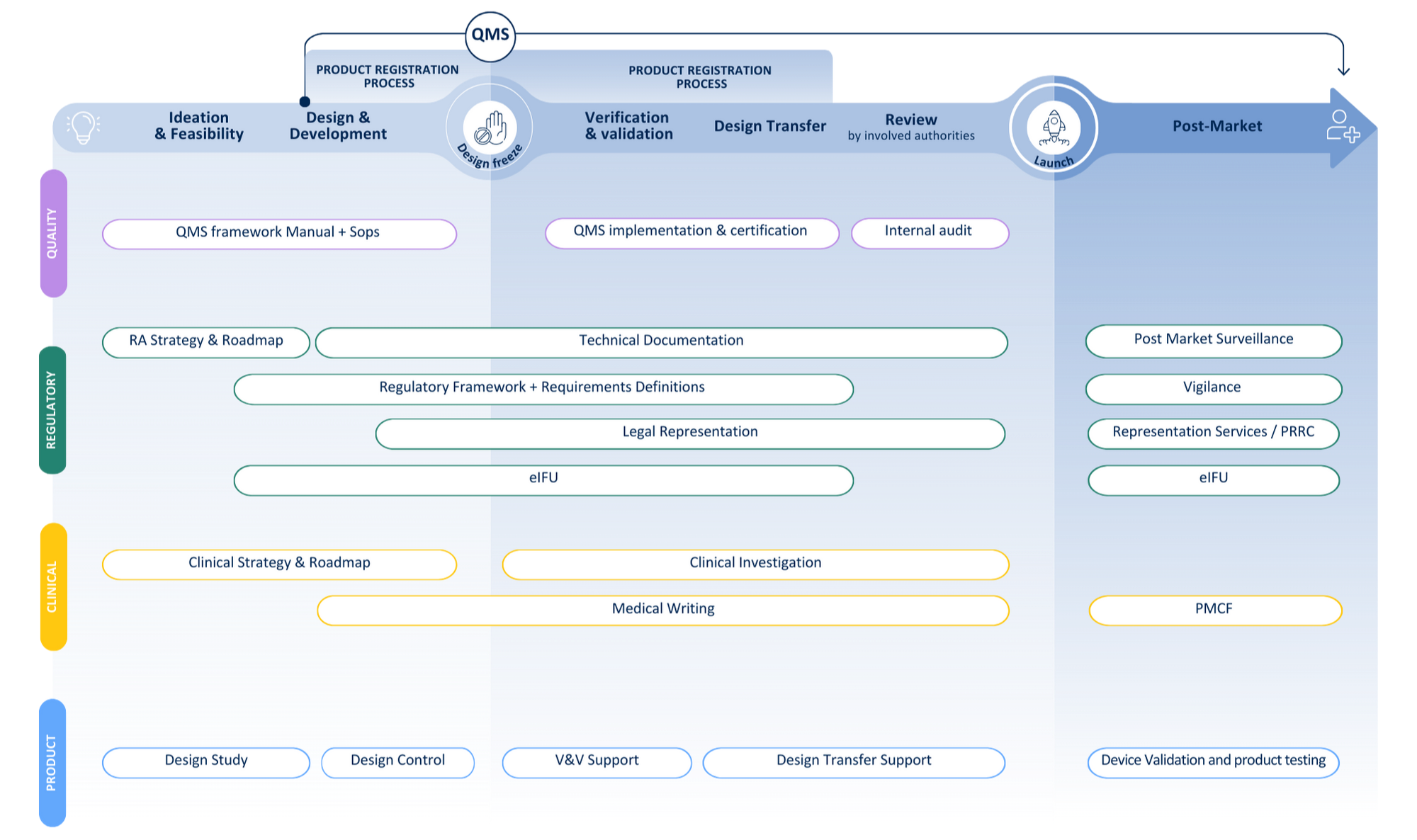
Recent client cases: combining compliance & clinical expertise
Explore how we helped two IVD manufacturers navigate complex study requirements and compliance frameworks — from IVDR and WHO alignment to full study sponsorship.
.jpg?width=800&height=450&name=Can%20IVDR%20Be%20Your%20Global%20Regulatory%20Compass%20for%20Market%20Entry%20(1).jpg)
Integrated IVDR & WHO Strategy
Class D Diagnostic Test
-
Challenge: Launch a Class D near‑patient and self‑test diagnostic under IVDR while also securing WHO prequalification—navigating two complex frameworks, tight timelines, and high sample‑volume demands.
-
Approach: Devised a unified IVDR/WHO protocol to eliminate duplication and minimize total samples; executed four targeted studies (combined clinical, IVDR near‑patient, IVDR self‑test, WHO self‑test); acted as legal representative to streamline EC/CA approvals and site management.
-
Result: Achieved first‑time‑right compliance for both IVDR CE‑marking and WHO‑PQ, cutting sample needs and accelerating time‑to‑market—paving the way for ongoing product collaborations.

Accelerated IVDR Market Access
Multiplex Respiratory Antigen Test
-
Challenge: Secure CE‑marking under IVDR for a rapid, multiplex respiratory antigen assay (professional + near‑patient use) ahead of the busy respiratory‑virus season, despite shifting classification rules across analytes.
-
Approach: Designed a lean, IVDR‑focused clinical performance plan; tapped our EU site network to fast‑track sample collection during peak season; handled ethical approvals, contracts, and logistics with agile oversight.
-
Result: Delivered robust clinical evidence on schedule, enabling CE‑marking in time for the season and empowering the client to launch their differentiated respiratory panel across Europe.

Multiplex STI PCR Test
-
Helped the client obtain IVDR CE‑marking for a fully automated multiplex real‑time PCR panel detecting eight low‑prevalence STI pathogens.
-
Crafted a lean clinical performance evaluation plan, optimized sample‑size calculations, and selected EU sites with access to the right patient cohorts.
-
Managed site initiation, monitoring, and data logistics across multiple countries, ensuring full GCP and IVDR compliance.
Result: First‑time‑right protocol approval and on‑schedule CE‑mark submission, establishing a repeatable framework for future IVDR filings.

Study Sponsorship Support
-
Took over IVDR performance study sponsorship for a U.S.-based IVD manufacturer.
-
Managed 8,000 samples across 100+ sites in 10 EU countries.
-
Provided support for study design, medical writing, GMS & GDPR compliance.
-
Coordinated EC/CA documentation and full Dx site monitoring.
-
Acted as legal representative throughout the study lifecycle.
Result: Streamlined execution, expert guidance, and long-term planning flexibility.

Combination of IVDR and WHO requirements
-
We helped launch a Class D product for point-of-care testing and self-testing in the European market, also achieving WHO-PQ approval.
-
We integrated IVDR and WHO requirements to reduce the number of samples needed and streamline processes.
- We organized four separate studies:
- Clinical study (IVDR + WHO)
- Point-of-care test (IVDR)
- Self-test (IVDR)
- Self-test (WHO)
- We acted as the legal representative for CE/CA approvals.
What you need to know
Understanding the role of clinical performance studies is crucial for any manufacturer of In Vitro Diagnostic (IVD) devices. Below, we answer some of the key questions about why these studies matter and how we support you through the process.
What are IVD clinical performance studies?
IVD clinical performance studies are essential in assessing and confirming the safety and performance of In Vitro Diagnostic Medical Devices (IVD). The IVD Regulation (EU) 2017/746, ISO20916:2024, and the World Health Organization (WHO) have defined stringent requirements that manufacturers must comply with.
We offer clinical performance studies in collaboration with renowned clinical laboratories, blood banks, and state-of-the-art private laboratories across Europe. As a sponsor, you can be confident that we will handle every aspect of the clinical performance study for your IVD, leaving you free to focus on your core business activities.
Why are these studies important?
- Clinical Performance Studies are not just a regulatory requirement – they’re critical for proving that your IVD device performs reliably and safely under real-world conditions.
- These studies help safeguard the health and safety of patients and users, while also ensuring that your device meets the necessary criteria for market approval.
- With evolving regulations and growing industry standards, having accurate clinical evidence is essential to gaining regulatory approval and ensuring market success for your IVD devices.

Get in touch
As a sponsor, you can be confident that we will handle every aspect of the clinical performance study for your IVD, leaving you free to focus on your core business activities.

Related resources


















.jpg)








.jpg)