Artificial intelligence (AI) and machine learning (ML) are on the rise in our society, changing the landscape of our daily lives, just think about face recognition and Google Lens applications that you use on your smartphone.
But what exactly do we think of when we talk about AI and ML in the context of the medical device (MD) and the in vitro diagnostic medical device (IVD) industry? And as calls grow for more regulatory oversight on AI/ML technology, how should this apply to MDs and IVDs?
In this blog post, we’ll illustrate how AI/ML applications are starting to boom in the medical device and IVD industry, and using 3 simple examples, we’ll show how AI/ML can help
-
to improve results generated by existing hardware solutions,
-
increase efficiency in medical laboratories,
-
and facilitate at-home tests through smartphone applications.
The AI/ML landscape in the MD/IVD industry
Until recently, it was difficult to put a figure on the number of medical devices and IVDs that incorporate artificial intelligence or machine learning-driven software, but now we can – at least if we take the USA as representative geography.
In October 2022, the FDA published a list of cleared/approved Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices and the Administration will keep on updating this list, which proves to be a treasure trove of information.
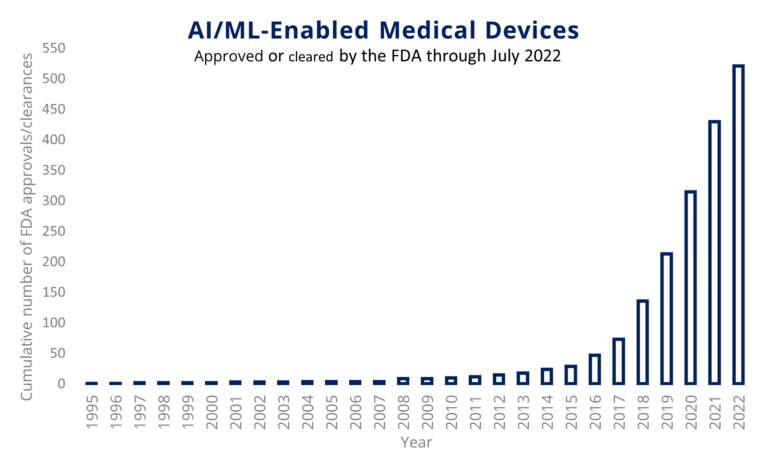
As you can see from the figure below, over 500 machine learning or other artificial intelligence medical devices have now received FDA clearance or approval, the first ones even preceding the year 2000!
However, the industry must have really started ramping up from 2010 onwards as approvals/clearances show an exponential increase starting in 2016.
Now, what is AI/ML used for in the medical field? A first observation is no surprise: MDs are vastly outweighing IVDs. The therapeutic area of radiology alone represents a whopping 75% of all devices and is supplemented with cardiovascular and neurology, these top 3 areas comprise 90% of all devices.
The first IVD-related therapeutic area is hematology, currently slightly below 3% of devices. Of course, the market share of MDs is far larger than that of IVDs, but some therapeutic areas are simply providing a more natural habitat for AI and ML technology to be incorporated in.
As we’ll see in the examples below, imaging is an important feeding ground for medical AI/ML solutions.
Three examples to illustrate how AI/ML supports the medical field
Of course, the list contains the well-known – if not controversial – 23andMe Personal Genome Service, but here we will show how AI/ML is becoming more and more common in the daily healthcare environment.
CT imaging medical device
As a first example, let’s take a look at a typical hospital MD application: X-ray imaging. Interpreting computed tomography (CT) images requires years of medical training but still, radiologists depend on the quality of the images they get.
As an example, obesity decreases image resolution. The GE Healthcare Deep Learning Image Reconstruction software, approved by FDA in July 2022, tackles this issue.
This X-ray data reconstruction method produces high-quality CT images through a deep neural network (DNN), which was trained with high-quality data sets to learn how to differentiate noise from signals, and to suppress the noise (e.g., originating from fatty tissue) without impacting anatomical and pathological structures.
This a perfect example of how AI/ML improves the output generated by previously installed scanning devices.
IVD for professional laboratory use
We’ll stay in the professional laboratory use environment for our second example, but let’s turn to IVDs. The time when laboratory technicians manually performed blood cell differentials behind their microscopes is long gone, thanks to AI.
Already in 2001, CellaVision received FDA clearance for their first Automatic Hematology Analyzer and subsequent generations followed suit in 2004, 2008, 2011, 2017, and the latest in 2020.
With this technology, cell images from blood smears are analyzed automatically using artificial neural networks (ANN) trained to distinguish between classes of white blood cells, resulting in improved standardization and turn-around time for the medical laboratory.
IVD for self-test
Lastly, let’s go outside the walls of hospitals and laboratories. In June of 2022, Healthy.io received clearance for the Minuteful – Kidney Test, a urine self-test device to aid in the assessment of kidney health.
The test is comprised of a kit and a smartphone application, the latter consisting of both an app and a backend server that perform image analysis through machine learning algorithms.
The app can be used throughout a wide range of smartphones and operating systems. As such, this test is lowering the threshold for at-risk patients and increases the chances for timely treatment.
The regulatory framework for AI/ML-enabled medical devices / IVDs in the USA and EU
From these examples, it should be clear that artificial intelligence holds great promise to improve patients’ health and medical decision-making, provided of course that their safety and effectiveness can be guaranteed for users and patients.
While we’ve previously illustrated that there is a distinct but horizontal approach for validation of AI/ML-based systems, the question is whether the same tactic should be used for regulatory purposes i.e., regardless of whether such systems enable MDs/IVDs or support other (health-related) applications.
Let’s take a look at the US and EU to see how each geography is dealing with this challenge.
USA: FDA framework
In the USA, a specific regulatory framework for AI/ML-enabled MDs/IVDs is developing. As many types of software may meet the broad statutory definition of a medical device – and are therefore subject to FDA regulation – this definition has been modified in 2016 to exclude certain health-related software, allowing a focus on “true” medical device software.
Already in 2019, the FDA published a discussion paper, describing a potential regulatory approach to premarket review for AI/ML-driven medical device software and asking for stakeholder feedback.
In 2021, this feedback was addressed in an AI/ML-based medical device software action plan to further develop the regulatory framework, encourage harmonization of good machine learning practice, increase transparency to users and patients, support the development of methodologies to evaluate and address algorithmic bias, and develop an approach on real-world performance monitoring.
EU: Artificial Intelligence Act
The European Union seems to be taking an entirely different approach. While Artificial Intelligence and Machine Learning systems qualifying as (in vitro diagnostic) medical devices are already effectively regulated under the MDR and IVDR, new legislative action is pointing towards a horizontal approach, including all AI systems regardless of their application.
However, the industry is growingly concerned over this proposed Artificial Intelligence Act (AIA), fearing that introducing this new legislation on top of those and other existing regulations would lead to a “regulatory lasagne”, stifle innovation, and further drive companies away from the EU market.
Not only would the current proposal require double CE marking and conformity assessment, but it would also create overlapping technical documentation with divergent concepts and definitions.
A legal analysis, commissioned by the Dutch government, concluded that the proposed AIA does not provide solutions to existing issues but would rather overcomplicate regulatory hurdles for MD/IVD manufacturers.
Emergence of standards
There is also some progress in the initiation of industry standards. The recently published ISO/IEC 23053:2022 Framework for Artificial Intelligence (AI) Systems Using Machine Learning (ML) is still quite general but can be helpful to describe a generic AI system using ML technology.
As more than 20 standards on AI/ML and digitalization are in the making, certainly keep an eye on the ISO/IEC JTC1 technical committee in general and specifically on the AI-dedicated subchapter SC42.
AI and ML are technologies that are here to stay, including in the medical device/IVD sector. In fact, the FDA’s recently published list shows that while we are only at the beginning of this healthcare revolution, AI/ML is already providing practical solutions for different target patient groups and usage environments.
Several governments are also taking legislative action to maintain regulatory oversight, but while the US is developing a specific framework for IVD/MD, the EU is preparing a holistic approach that – if approved as currently proposed – will present significant challenges to the industry.
At the QbD Group, our consultants are always on top of the latest trends and regulatory initiatives in the digital health landscape. Don’t hesitate to contact us to see how we can help you bring your AI/ML-based medical devices or IVDs to market!







.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)













.jpg)







