Plasmids are used in various research and biotechnology applications, such as gene therapy, vaccine development, and recombinant protein production.
Whether you are a plasmid manufacturer or user, you need to have specific knowledge of plasmid manufacturing and regulations before going to market.
This blog discusses the core principles of plasmid manufacturing and the regulations involved.
What are plasmids?
Plasmids are small, circular pieces of DNA found in bacteria and other organisms. They can replicate independently of the host genome and carry genes that provide the host with certain advantages, such as resistance to antibiotics or the ability to metabolize specific nutrients.
The exact process by which plasmids are created can vary depending on the circumstances, but in general, it involves the following steps:
1. Acquisition of foreign DNA
Plasmids can acquire foreign DNA through a variety of mechanisms, such as transformation (uptake of naked DNA from the environment), transduction (transfer of DNA via bacteriophages), or conjugation (direct transfer of DNA between bacteria).
2. Integration of foreign DNA
Once the bacterium has acquired the foreign DNA it needs to integrate it into the plasmid. This can happen through a variety of mechanisms, such as homologous recombination or transposition.
3. Replication
Plasmids can replicate independently of the host genome, which means that they can exist in multiple copies within a single bacterial cell. Plasmid stays in the bacterium and its descendants.
4. Maintenance
Plasmids must be able to maintain themselves within a bacterial population to persist over time. This can involve mechanisms such as selection for plasmid-containing cells, or the production of factors that ensure their own replication and stability.
The creation of plasmids involves a complex interplay between the plasmid itself and its bacterial host, as well as the acquisition and integration of foreign DNA.

Figure 1 – The general structure of a plasmid (source reference 1)
Increasing the quantity: Plasmid manufacturing
Plasmid manufacturing refers to the process of producing enormous quantities of a specific plasmid DNA sequence for use in research or biotechnology applications. The process involves several steps, which may vary depending on the specific plasmid and the intended use.
There are multiple methods for plasmid banking, including:
1. Design
The first step in plasmid manufacturing is to design the sequence. This involves selecting the appropriate genetic elements (such as promoters, regulatory sequences, anti-biotics resistance gene, and selectable markers) and arranging them in a way that allows the plasmid to express the desired gene(s) and confer the desired traits to the host cell.
2. Cloning
Once the sequence is designed, it must be cloned into a suitable vector, such as a bacterial plasmid or a viral vector. This is usually done using recombinant DNA technology, which involves cutting the plasmid and vector DNA with specific enzymes and then ligating them together.
3. Host Cell Transformation
The resulting recombinant plasmid DNA is introduced into a host cell, usually a bacterial cell such as E. coli. The host cell is transformed with the plasmid using methods such as electroporation, heat shock, or chemical transformation. Colonies are created of the plasmid containing bacteria for the creation of master cell banks (MCBs), see section below “Building the reserves: Plasmid banking”. Mostly, working cell banks (WCBs) are created to allow for the manufacturing of a single plasmid.
4. Amplification
Once the plasmid has been introduced into the host cell (and the MCB and/or WCB is created), it must be amplified to produce enormous quantities of the DNA sequence. A vial from the MCB or WCB is thawed and further processed to begin the amplification of the plasmid. A variety of methods can be used, such as fermentation or shaking flask culture. During this step, the host cells grow in a nutrient-rich medium that allows them to multiply and produce multiple copies. Since the plasmid has an anti-biotics resistance gene, the medium contains anti-biotics such as Kanamycin so that only the plasmid containing E. Coli can grow.
5. Purification
Once the host cells have grown and produced the plasmid DNA, the DNA is extracted and purified from the cells and other cellular components. This usually involves several rounds of purification, such as alkaline lysis, column chromatography, and precipitation.
6. Quality Control
Finally, the purified plasmid DNA is subjected to quality control tests to ensure that it is pure, intact, and free of contaminants. These tests may include gel electrophoresis, spectrophotometry, and sequencing.
The resulting plasmid DNA can be used in a variety of research and biotechnology applications, such as gene therapy, vaccine development, and recombinant protein production.

Figure 2 – Plasmid manufacturing overview (source reference 2)
Building the reserves: Plasmid banking
However, before amplification during fermentation, a plasmid-containing colony is selected for the creation of a master cell bank (MCB). This is called plasmid banking. Plasmid banking refers to the process of preserving and storing plasmids for future use. This is important for research and biotechnology applications, as it allows scientists to access and use specific plasmids at a later time, without having to repeat the entire plasmid manufacturing process.
There are multiple methods for plasmid banking, including:
1. Freeze-drying
In this method, the plasmid DNA is dissolved in a buffer solution and then freeze-dried to remove all water. The resulting powder can be stored at room temperature and reconstituted with water when needed.
2. Glycerol stocks
In this method, the plasmid DNA is mixed with a cryoprotectant such as glycerol, and then frozen at -80°C. This allows the plasmid to be stored for extended periods of time without degradation.
3. Ampoule method
In this method, the plasmid DNA is mixed with a cryoprotectant and then stored in small glass ampoules, which are sealed under vacuum and then frozen at -80°C.
4. Bead-based storage
In this method, the plasmid DNA is immobilized on small silica or glass beads, which are then stored in a desiccated or nitrogen environment. This method allows for easy and efficient storage and retrieval of plasmids.
Regardless of the storage method used, plasmid banking requires careful documentation and tracking to ensure that the plasmids can be easily located and retrieved when needed. This includes information on the sequence, the date of creation, the storage location, and any relevant quality control data.
Regulatory requirements for manufacturing
In 2018, the EMA released a specific guideline on the quality, non-clinical, and clinical aspects of gene therapy medicinal products and defined plasmids as starting materials (4).
The principles of Good Manufacturing Practice (GMP) for the manufacturing of starting materials of biological origin used to transfer genetic material for the manufacturing of ATMPs require that plasmid manufacturing processes be well-characterized, controlled, and validated to ensure consistency and quality of the final product. The manufacturing process should be designed to minimize the risk of contamination or degradation of the plasmid during all stages of production, from cell banking to final purification.
The EMA also requires testing of plasmids to ensure their identity, purity, potency, and safety. This includes testing for the presence of impurities such as residual host cell DNA, endotoxins, and adventitious agents, as well as assessing the stability and potency of the plasmids.
Furthermore, EMA released a document about the questions and answers on the principles of GMP for the manufacturing of starting materials of biological origin used to transfer genetic material for the manufacturing of ATMPs in 2021(5). In this document, the EMA states that a risk-based assessment is needed to determine the GMP principles for starting materials in the production of ATMPs. Furthermore, a Qualified Person (QP) is not required in connection with the manufacturing of starting materials.
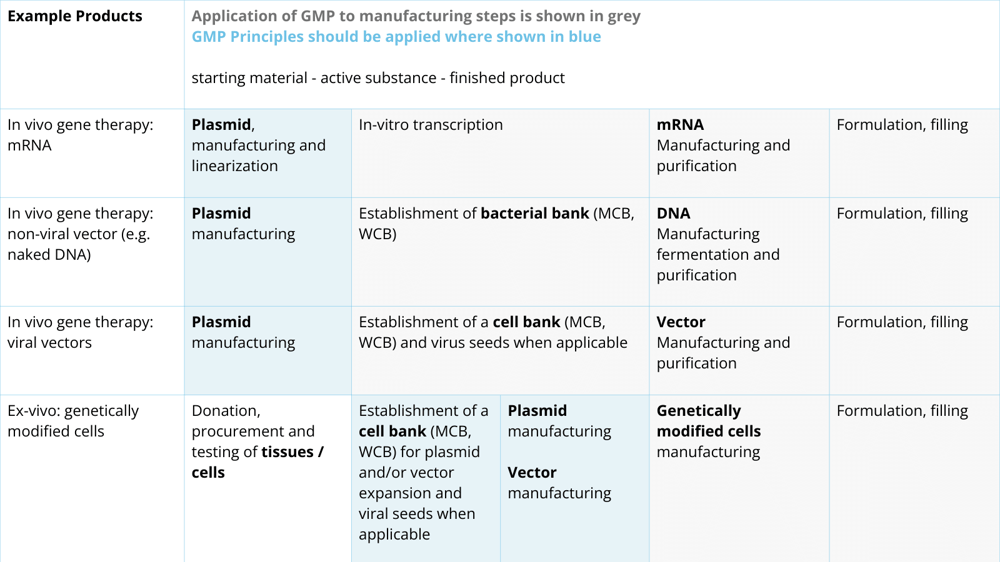
In this document, the EMA released a table (see Figure 3) to support the understanding of the GMP requirements for various products using plasmids. The light grey fields show where the principles of GMP should be applied, and the dark grey fields show where full GMP procedures should be applied. The EMA speaks about using a risk-based approach to determine which level of GMP is required and that this should be proportional to the impact of the starting material on the quality, safety, and efficacy of the finished product.
As can be concluded from the table in Figure 3, ATMP manufacturers should comply with GMP procedures, but the plasmid manufacturers should follow the principles of GMP. Therefore, it is important that ATMP manufacturers qualify plasmid manufacturers to ensure that they are supplied with high-quality plasmids suitable for the intended use in ATMP production.
For the US, the FDA, Food and Drug Administration, published a guideline in 2020 providing the Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs) (6). The classification of plasmids used in cell and gene therapy processes remains a more ambiguous area in terms of their classification (i.e., ancillary material, raw material, starting material, or drug substance intermediate).
The plasmids should be created from qualified banks and the information about the materials used in the creation of these banks should be documented in the IND. Furthermore, for the early phase, the creation of master cell banks is not necessary. However, all the information about the starting materials to create the bank should be described in the IND.
To determine the quality of the plasmids, they should be tested for, but not limited to, purity, endotoxin, sterility, and identity. Also, the master cell bank should at least be tested for the presence of plasmid, bacterial host strain ID, cell count, plasmid ID, plasmid sequence, and host strain purity. Further analytical tests might be necessary depending on the requirements for the use of the plasmid.
Conclusion
Plasmid manufacturing is a complex process that involves several steps, including design, cloning, host cell transformation, plasmid amplification, purification, and quality control.
The resulting plasmid can confer a wide range of research and biotechnology applications, such as gene therapy, vaccine development, and recombinant protein production. One part of the manufacturing process is plasmid banking which allows the access and use of specific plasmids at a later time.
Different guidelines exist to regulate the use of plasmids in gene therapy products and ensure that they are used safely and effectively for the benefit of patients.
The EMA’s guideline of 2018 and the follow-up questions and answers of 2021 (4,5), clarified the principles of GMP in the manufacturing of plasmids and described the risk-based approach in determining the level of GMP based on the impact of the starting material in the quality, safety, and efficacy of the finished medicinal product.
The ATMP manufacturer should comply with GMP procedures, but the plasmid manufacturer should comply with the principles of GMP. It is therefore important that the ATMP manufacturer qualifies the plasmid manufacturer to ensure the quality of the plasmid and therefore the final product.
The FDA on the other hand defined plasmids as intermediate products and defined no specific GMP requirements for manufacturing (6). It states that plasmids need to be created from qualified banks and that all the information about the materials used in the creation of these banks should be listed in the IND. Furthermore, the guideline provides a recommended list of analytical tests to determine the quality of the plasmid and the plasmid banks.
- https://www.genome.gov/genetics-glossary/Plasmid
- Regulatory & supply chain implications for plasmids as critical starting materials in the manufacture of viral vector gene therapy products by Melissa Gosse, Cameron Jones, Desyree Jesus & Susan D’Costa
- Guidelines on Good Manufacturing Practice specific to Advanced Therapy Medicinal Products
- European Medicines Agency, 22MAR2018, EMA/CAT/80183/2014, Committee for Advanced Therapies (CAT), Guideline on the quality, non-clinical and clinical aspects of gene therapy medicinal products
- Questions and answers on the principles of GMP for the manufacturing of starting materials of biological origin used to transfer genetic material for the manufacturing of ATMPs
- Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs)







.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)




%20Checklist.jpg)








.jpg)




.jpg)
.jpg)

.jpg)


.jpg)
.jpg)
.png)