Immunotherapy has become an important therapeutic alternative to surgery, chemotherapy, and radiation in the fight against cancer. Oncolytic viruses and cellular immunotherapy are creating significant new potential in the fight against cancer.
In this article, we discuss the working mechanism, risks, and specific biosafety requirements to be considered for oncolytic viruses. (For cellular immunotherapy, please see our CAR-T blog post.)
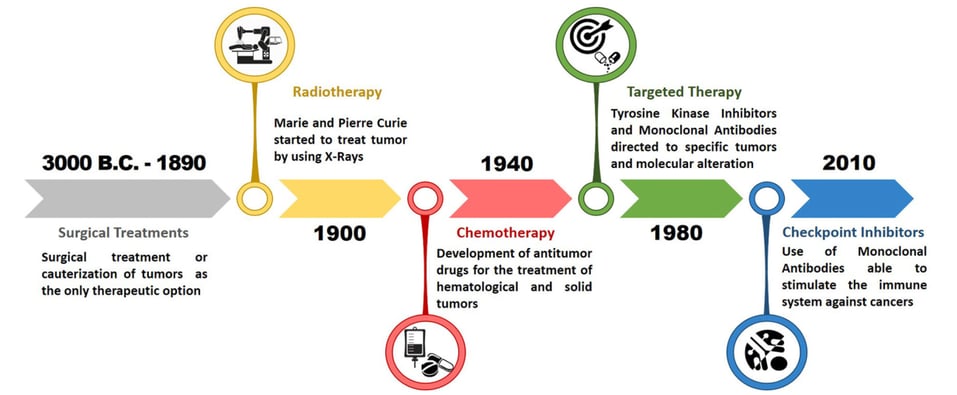
Figure 1 – Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium: Falzone Luca, Salomone Salvatore and Libra Massimo, Frontiers in Pharmacology Vol:9 (2018).
What are oncolytic viruses and how do they work?
Viruses are selected based on their inherent oncotropic nature; these viruses are wild-type viruses such as Parvovirus. Other viruses are genetically modified to selectively target tumor cells.
Genetic modification offers several advantages, namely fewer side effects by reducing pathogenicity to non-cancer cells and preventing an antiviral immune response. Oncolytic viruses act on two main mechanisms, tumor cell lysis and/or anti-tumor immunity.

Figure 2 – Oncolytic Viral Therapy and the Immune System: A Double-Edged Sword Against Cancer: Marelli Giulia, Howells Anwen, Lemoine Nicholas R., Wang Yaohe. Frontiers in Immunology, Vol:9 (2018).
How are oncolytic viruses used and what are the advantages?
Oncolytic virotherapy can be seen as the third revolution of tumor treatment after traditional chemotherapy and targeting therapy. OVs are being researched in the treatment of cancer to replace or combine with current treatment options, such as surgery, chemotherapy, hormonal therapy, targeted therapy, or radiation therapy. Those therapies lead to limited long-lasting responses in a large majority of patients with advanced cancer.
Oncolytic virotherapy has many advantages over other tumor immunotherapies, including precise targeting, high lysis efficiency, and low cost. Therapies can be administered by various routes, including direct intra-tumoral administration, which is the most common. Intravenous injection, intraperitoneal, intrathecal, or subcutaneous administration are also possible.
What are the risks and biosafety requirements of oncolytic viruses?
In addition to the benefits, there are some challenges and risks associated with the use of OVs to treat cancer. EudraLex Volume 4 Part IV, GMP for ATMPs, identifies some specific precautions for viral vector production. Specific focus on cross-contamination and segregation is highlighted. In the manufacturing plant a biosafety responsible will be evaluating the safety and precaution measurements of working with viral and genetically modified organisms.
Biosafety levels
When working with potentially infectious agents, safety measures must be taken to reduce the risk of exposure to the laboratorian and contamination of the work environment and community. This is known as biosafety.
The Centers for Disease Control and Prevention (CDC) has specified four different types of biosafety levels (BSL) in the CDC guidance, ranging from BSL-1 to BSL-4. These levels aligned with the containment levels of the HSE (Health and Safety Executive in the UK) and EU regulations (Directive 2000/54/EC).
The level is determined by several factors including infectivity, severity of disease, transmissibility, and the nature of the work performed. The origin of the biological agent and the route of exposure are also important to consider. All these elements combine to determine what BSL is needed.

Figure 3 – The Centers for Disease Control and Prevention (CDC) has specified four different types of biosafety levels (BSL) in the CDC guidance, ranging from BSL-1 to BSL-4.
Each BSL has its own containment controls required for laboratory practices, safety equipment and facility construction. Each microbiology laboratory follows the standard microbiological practices; this is always required. Additional requirements are added depending on the level.

Current therapies and clinical trials
The development of OVs is still in the early stage. Many viral oncolytic therapies are currently in clinical trials, with many showing promising results. Most therapies include the use of genetically engineered OVs alone or in combination with other therapies. Oncolytic virotherapy may be effective as a stand-alone therapy, but combination therapies with radiotherapy, chemotherapy or other immunotherapies are probably necessary to gain full potential. The most widely studied viruses in clinical trials up until now were adenoviruses and herpesviruses.
Imlygic®, was the first oncolytic immunotherapy approved by the FDA and EMA in 2015. The active substance in Imlygic®, talimogene laherparepvec, is derived from a weakened herpes simplex virus 1 (the cold sore virus). This virus has been modified so it can infect and multiply inside melanoma cells. Imlygic uses the melanoma cells’ own machinery to multiply, eventually overwhelming melanoma cells and killing them. Although Imlygic can enter healthy cells, it is not designed to multiply inside them.
Development of these products is challenging and linked to viral safety. Minimizing the viral replication of OVs in healthy cells is one of the main concerns in the first stages of clinical trials. Another aspect is the prevention of integration of the viral vector into the host genome. Vector design and engineering are therefore very important.
Conclusion
Oncolytic virotherapy offers promising therapy for cancer treatment. Do you need help with ATMP-related problems? Do not hesitate to contact our ATMP experts.
We are happy to help you with facility design, QC testing and regulatory advice during your oncolytic virus development process.
- Oncolytic viruses for cancer immunotherapy | Journal of Hematology & Oncology | Full Text (biomedcentral.com)
- Frontiers | Oncolytic Virotherapy: From Bench to Bedside (frontiersin.org)
- Definition of oncolytic virus – NCI Dictionary of Cancer Terms – NCI
- Oncolytic viruses: a new class of immunotherapy drugs | Nature Reviews Drug Discovery
- ICH Considerations: Oncolytic Viruses (europa.eu)
- Frontiers | Delivery and Biosafety of Oncolytic Virotherapy (frontiersin.org)
- Frontiers | The Oncolytic Virus in Cancer Diagnosis and Treatment (frontiersin.org)
- Therapy with oncolytic viruses: progress and challenges | Nature Reviews Clinical Oncology
- CDC LC Quick Learn: Recognize the four Biosafety Levels
- 2017_11_22_guidelines_gmp_for_atmps_0.pdf (europa.eu)
- Oncolytic-virus-design.pdf (idt-biologika.de)
- Advances in oncolytic virotherapy | Communications Medicine (nature.com)







.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)




%20Checklist.jpg)








.jpg)




.jpg)
.jpg)

.jpg)


.jpg)
.jpg)
.png)