The UK Medicines and Healthcare Products Regulatory Agency (MHRA) has been conducting Good Pharmacovigilance Practice (GPvP) inspections since 2003. Any Marketing Authorisation Holder (MAH) or marketing authorization applicant is subject to a GPvP inspection to determine their compliance with the necessary pharmacovigilance duties required, in order to monitor the safety of medicines to the public in the United Kingdom (UK).
Inspections are typically scheduled by Pharmacovigilance System, product or post-authorisation safety study, rather than by MAH. To establish safety and compliance, there must be a Pharmacovigilance System in place for each MAH which describes in detail, the legal roles and responsibilities required to demonstrate the safety of the authorised medicinal product, in accordance with any changes to the risk-benefit ratio of the product.
The MHRA GPvP Symposium was held on 28th February 2024, where a range of topics were discussed to give an insight into how the MHRA achieved their goal of safeguarding the health of the public.
In this blog, the focus is more on the inspection metrics, to highlight the key areas where MAHs can continue to improve their PV system and prevent those critical, major or minor findings!
Types of Inspections
The objective of routine PV inspections is to assess whether the MAH can identify, characterise and report new or changed risks associated with their medicinal products.
Routine inspections can be either initial or re-inspections and are scheduled as part of the MHRA’s national inspection program according to a risk-based approach in line with the Good Pharmacovigilance (GVP) Module III. The MHRA will provide notice to the MAH before the inspection for preparedness.
Depending on the findings, the MAH may be subject to a re-inspection, otherwise, if there are no critical findings, there is no specific timeline by which an MAH must be inspected again, and this will be determined using a risk based approach.
Triggered inspections, also known as ‘for cause inspections’ can be scheduled due to intelligence or a previous critical finding. This includes if the MHRA have been informed of specific risk information regarding the MAH and its authorised products. The following breaches of the GPvP also call for a triggered inspection:
-
a whistleblower
-
other MHRA departments
-
another regulatory authority
For these types of inspections, very little or no notice is given in advance.
MHRA GPvP Inspection Metrics for 2022/2023
There were 17 PV inspections conducted in 2022/2023 which included routine inspections and triggered inspections.
A significant number of inspections conducted during this period were ‘for cause – intelligence’ inspections. The graph below shows the breakdown of findings issued by the MHRA along with the inspection type.

Source: Medicines and Healthcare products Regulatory Agency
The majority of critical findings were issued for routine initial inspections, followed by routine re-inspection and ‘for cause – intelligence’ inspections. The major and minor findings for ‘for cause – previous critical’ inspections are significantly lower than other inspection types, which is likely due to the limited scope for these types of inspections.
A robust Quality Management System (QMS) is required within a Pharmacovigilance system for the MAH. Though, the QMS may not be inspected directly by the MHRA, the majority of the major and minor findings were found in this area due to the QMS underpinning the surrounding PV topics. Two critical findings were issued for Risk Management and one for Ongoing Safety Evaluation which is illustrated below.

Source: Medicines and Healthcare products Regulatory Agency
During the reporting period of 2022/2023, four critical findings were issued and described below:
- Signal Management
- Maintenance of Reference Safety Information (RSI)
- Additional risk minimisation measures (aRMMs)
- Additional risk minimisation activities
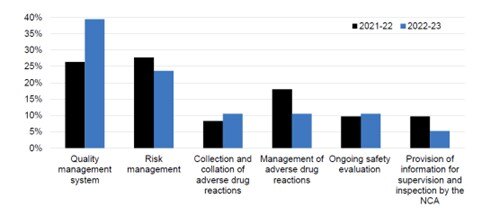
The number of inspections from the previous reporting period (2021/2022) has reduced from 32 to 17, however, the mean number of inspection days has remained stable at around 9 days per inspection. The following topics remain the highest in terms of findings during the inspections:
- Risk Management
- Ongoing safety evaluation
- Quality management system
Source: Medicines and Healthcare products Regulatory Agency
Remote Compliance Assessment Pilot
A Remote Compliance Assessment was piloted in the GPvP team, and it is a new approach to following significant findings from previous inspections. Typically, a re-inspection would be conducted following a major or critical finding to ensure that the remediation activities were adequately implemented. However, this new approach replaces the need for a full re-inspection, under specific circumstances.
RCA
- Significant finding in one main topic
- Limited but targeted scope
- Always remote
- Document-based review of remediation is feasible.
Re-inspection
- Significant findings in multiple topics
- Remote inspection is not feasible
- Increased scope
- Remediation activities are complex or require interviews.
As there will be one focused topic to be considered, a full inspection plan, company presentation and pre-scheduled interviews will not be required. Instead, a focused RCA report form and a short introductory meeting will be held and a CAPA will only be raised if the finding is critical or major.
Conclusion
The GPvP team at the MHRA continue to improve their process of how inspections are conducted to ensure that it is time and cost-effective for all parties. It is crucial to ensure that the foundations, such as a QMS are in place for an MAH to establish and maintain the PV activities necessary for a fully compliant and risk-based approach for medicinal products.
Our Vigilance department at QbD Group will collaborate closely with you to understand your business needs and objectives and then offer specialised solutions to assist you in accomplishing them. With our expert guidance, you can confidently navigate pharmaceutical regulations.







.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)




%20Checklist.jpg)








.jpg)




.jpg)
.jpg)

.jpg)


.jpg)
.jpg)
.png)