Digital health in pharma is not just an emerging trend; it’s shaping the future of the pharmaceutical industry. This evolution is altering the healthcare landscape, encompassing everything from traditional pharmaceuticals to cutting-edge BioPharma, as well as Medical Devices (MDs) and In-Vitro Diagnostics (IVDs). Previously, we dissected the digital health arena into 3 core domains: Pharma, Clinical, and MDs/IVDs, and identified 4 foundational pillars: blockchain, ML/AI, cybersecurity, and digitalization.
In this blog post, we delve into how digital health is being adopted within the Pharma sector, examining its role in the future of pharma. We’ll explore established applications, such as anti-counterfeiting and automated vision inspection, highlighted in our earlier roadmap. But the realm of digital health is vast and constantly evolving, so we’ll also venture into the latest advancements and innovations that are reshaping pharmaceuticals in the digital age.
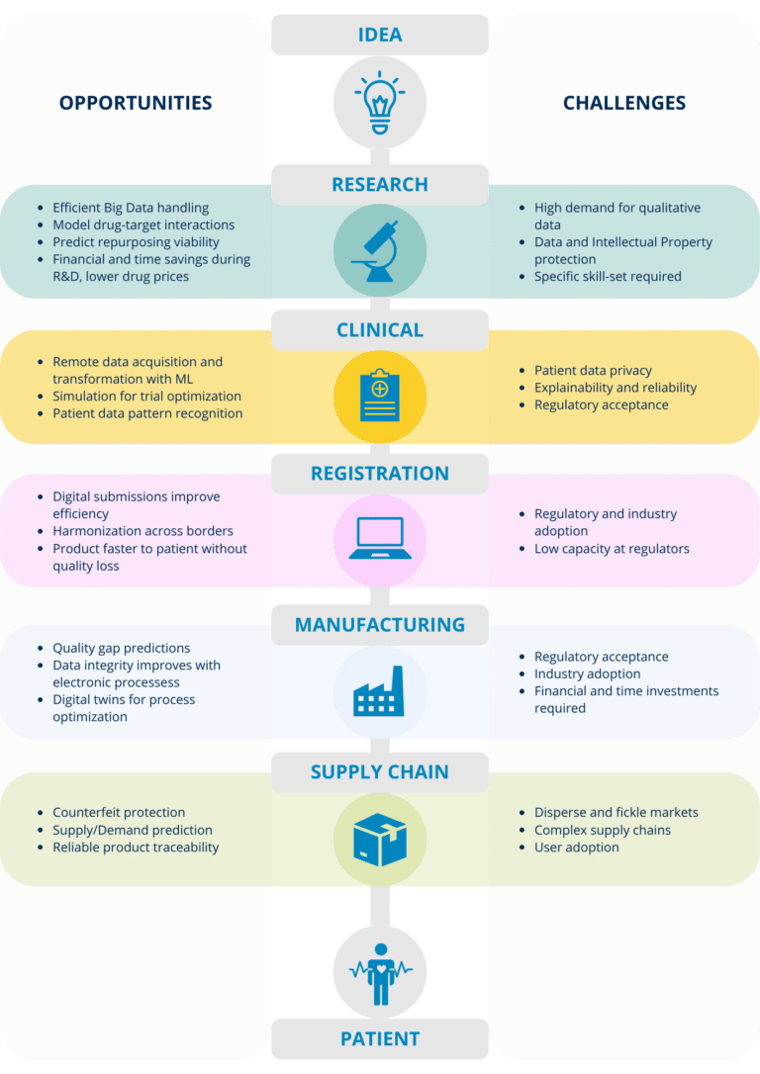
Figure 2 – Pharmaceutical lifecycle from idea to patient.
We’ll present the digital health journey the industry is facing and how different companies are already targeting challenges and harnessing opportunities brought by digital applications. An infographic summarizing this blog post and presenting the opportunities and challenges is presented in Figure 3.
Drug discovery & preclinical development
.png?width=345&height=191&name=Digital%20health%20in%20pharma%20(2).png)
The cost of developing a single new drug has recently been estimated to be more than $ 1 billion, with average development times exceeding a decade. This inefficiency stems mainly from low success rates. Hence, unsurprisingly, there is a substantial drive to improve efficiency within this preclinical part of the Pharma and Biotech industries. A challenge perfectly suited for modern-day digital technologies.
Oversimplifying it a bit, we could summarize pharmaceutical R&D in the following three steps:
-
a disease or affliction is selected
-
for which a drug or treatment is then developed
-
of which efficacy and safety are then assessed through clinical studies.
Here, we’ll focus on the opportunities that digital health can create in these preclinical stages.
AI/ML technologies can be wielded to
- learn from mistakes and breakthroughs of previous studies,
- structure big uncoherent datasets,
- predict the interaction of a new molecule with targets within the patient’s body,
- analyze patient data for better disease understanding,
- or select niche populations of patients for personalized treatments.
Combining the power of AI/ML with pre-clinical researchers’ expertise will effectively speed up and improve success rates of drug discovery and development. Thus, bringing new or improved therapies to the right patients sooner and cheaper, without sacrificing quality or safety.
It is important to mention that all AI approaches generate an incredible demand for data. The collection, storage, and handling of large amounts of data, in turn, also demands novel approaches related to data generation, data mining, data analysis, privacy, and cybersecurity. Blockchain technology could be the solution to ensure validity, security, and traceability when sharing valuable pre-clinical data and sensitive patient data.
Application 1: Insilico – Drug Discovery Pipeline
Companies like Insilico are developing an entire AI-powered digital drug discovery pipeline to aid in drug discovery; from target identification and analysis to drug candidate selection to clinical trial assistance and data analysis.
At the beginning of 2022, they announced the initiation of Phase 1 trials for a new drug product focused on a newly discovered antifibrotic target molecule, both thanks to their AI software. Moreover, they achieved this in under 30 months and for only 2.6 million dollars. Very promising figures.
Application 2: Drug repurposing
Some argue that in certain situations it may be unnecessary to re-invent the wheel and existing drug products may be multi-functional.
For example, Beck et al. leveraged a self-attention Drug Target interaction prediction model to predict the efficacy of currently available antiviral drug products to target infection by the SARS-Cov-2 virus.
By using a model to predict the efficacy of old products against a new pathogen, the need for de novo drug discovery can be postponed or eliminated altogether. This is especially useful in cases where time is of the essence, like the COVID-19 pandemic.
Clinical
Clinical trials are often still performed in conventional ways, even though there is a high potential for efficiency gains and an improved success rate through the implementation of digital health technologies.
Because we have defined the clinical trial stage as a separate domain, the specific applications and benefits of digital health in clinical trials were addressed in a separate blog post.
Registration
PDF-based application forms are being replaced by digital submission platforms by the EMA, FDA, … This improves the efficiency of the regulatory approval process from both the sides of the manufacturer as well as the regulator, thereby bringing the treatment to the patients sooner, potentially saving lives.
After approval, the product shall often be manufactured on an industrial scale and distributed to a large, dispersed set of patients. These stages in the product life cycle bring with them another set of challenges and opportunities where digital health will undoubtedly leave its mark in the upcoming years.
Industrial manufacturing
With industrial-scale processes, it is important to have a standardized and controlled production process that can continuously deliver the highest quality products, especially in the strictly regulated (Bio-)Pharma industry.
This extreme regulatory pressure on the industry can slow down innovation. Therefore, the ISPE has translated the principles of the Industry 4.0 revolution to the pharmaceutical sector under the Pharma 4.0 initiative. Pharma 4.0 has the best practices of health regulations already embedded into its core principles.
Currently, digitalization and automation are becoming more and more common across the industry to improve record-keeping, lab management, manufacturing processes, etc.
Terms such as ERP, MES, LIMS, and SCADA are no strangers to most pharmaceutical industry professionals. These are all digitized systems integral to Pharma 4.0 which enable production planning, plant- and lab management, process control, record keeping, etc.
But Pharma 4.0 goes further, it is a holistic view of the pharma industry that aims to leverage interconnected, smart, and digitized manufacturing processes to ensure a competitive advantage, in which the concept of Internet of Things (IoT) and Cyber-physical systems (CPS) will play a crucial role.
Application 3: Digital Twins
An interesting application already being used in other industries is Digital Twins. These digital replicas of vessels, filling lines, bioreactors, or even entire production floors, enable the simulation of the production processes.
This is useful for training new personnel or testing possible process optimizations prior to physical implementation.
Digital twins can also be used for continuous monitoring of the equipment for maintenance planning and early detection of defects. But such smart plant floors are still uncommon and require a lot more investment in continuous data collection, transfer, and analysis strategies.
Application 4: Deep learning for product inspection
Inspection of products is generally performed by automated vision models that classify products as acceptable or unacceptable. The unacceptable products are ejected and manually inspected.
Manual inspection is intensive and current automated inspection models lack the complexity to account for the highly variable nature of all thinkable defects a product could possibly exhibit.
Modern computer vision algorithms based on convolutional neural networks (CNNs) have the potential to significantly outperform currently applied models because of their extreme capacity to capture variability on the one hand, and their ability to be (semi-)continuously improved by learning from large amounts of data on the other hand.
Although this concept of learning and improving is a fundamental aspect of machine learning and deep learning, it raises questions in the pharma industry regarding the validation/qualification of such non-static systems. Read more about this here.
Supply chain
Once manufactured, a drug or treatment still requires distribution to a global and unpredictable market. Niche products need to be globally marketed to be financially viable, other products depend highly on variable demands steered by seasonal epidemic periods or unpredictable disease prevalence, and products can have very limited expiry dates or very critical storage conditions.
Together, these unique challenges make the pharmaceutical supply chain extremely complex and render standard solutions from other industries unusable.
Ideally, a company always has the right amount of stock at the right location and as little product waste as possible. These desires do not go well together in case of limited shelf life and unpredictable demand. What if we could actively track the consumption of our product throughout the entire supply chain and predict shortage and possible wastage using smart models?
When a single salable unit of a drug rolls off the production belt in a pharmaceutical company, it is generally labeled with a serial number, i.e., a unique identifier. This process, called serialization, generally encodes the origin, batch number, and expiry date of the product.
It encrypts data in a serial number so that it can later be retrieved for reliable product tracking and identification. Effective serialization and the data it could generate is a powerful tool for supply chain management and shortage prevention, and it also is the pharma industry’s best protection against counterfeiting
Although serialization is already common practice, counterfeiting remains an increasing problem. Blockchain technology could render serial number falsification almost impossible, and thus aid in the fight against counterfeiting. It achieves this by using an encryption method called a ‘Hash function’ that creates a ‘hash’-code based on certain input data, which is very hard to decrypt.
Moreover, product traceability can be ensured by including the previous hash code in the input data used to generate the following hash, because this creates a one-directional chain of blocks of information, hence “block-chain”.
Application 5: The Pharmaledger initiative
Pharmaledger is a partnership between universities, big pharma, hospitals, patient organizations, and tech companies that aim to build a blockchain-based applications platform for manufacturers as well as patients.
Blockchain-based serialization combined with a highly accessible digital platform allows product traceability throughout the entire distribution supply chain from manufacturing plant to patient.
Consequently, it improves control over product distribution, can generate product demand data, makes recalls more feasible, prevents counterfeit products from entering the market, simplifies adverse event reporting from patients, simplifies information sharing, etc.
In other words, effective serialization from production plant to patients is valuable for both big pharma as well as patients and their safety.
Conclusion: digital health in pharma is on the rise
Digital health in Pharma is on the rise and is a serious candidate to be the next key competitive advantage for pharmaceutical companies.
By optimizing the different product lifecycle stages with digital health technologies, new products can be brought all the way to the patient safely, fast, and at a lower cost.
Applications such as the Pharmaledger will give patients easier access to updated drug product information and a means to verify the legitimacy and origin of the product.
Digital improvements in the R&D and manufacturing phases will reduce costs and improve efficiency and quality, lowering prices for patients and bringing therapies to people in need sooner.
Implementing all the discussed applications in practice will be a costly and time-consuming endeavor, but the rewards will be proportionate, both for industry and patient.
- http://proceedings.mlr.press/v106/shin19a.html?ref=https://githubhelp.com
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7118541/
- https://link.springer.com/content/pdf/10.1007/s11030-021-10217-3.pdf
- https://link.springer.com/content/pdf/10.1208/s12248-021-00644-3.pdf
- https://www.nature.com/articles/s41746-019-0148-3
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7577280/
- https://chemrxiv.org/engage/api-gateway/chemrxiv/assets/orp/resource/item/60c7486cbb8c1ac5453dac67/original/potential-covid-2019-3c-like-protease-inhibitors-designed-using-generative-deep-learning-approaches.pdf
- https://insilico.com/phase1
- https://www.nature.com/articles/d41586-020-03348-4
- Whitepaper: Exploring the unexplored, drugging the undrugged (cyclicarx.com)
- https://www.icr.ac.uk/blogs/the-drug-discoverer/page-details/reflecting-on-deepmind-s-alphafold-artificial-intelligence-success-what-s-the-real-significance-for-protein-folding-research-and-drug-discovery#content_wrap







.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)











.jpg)







