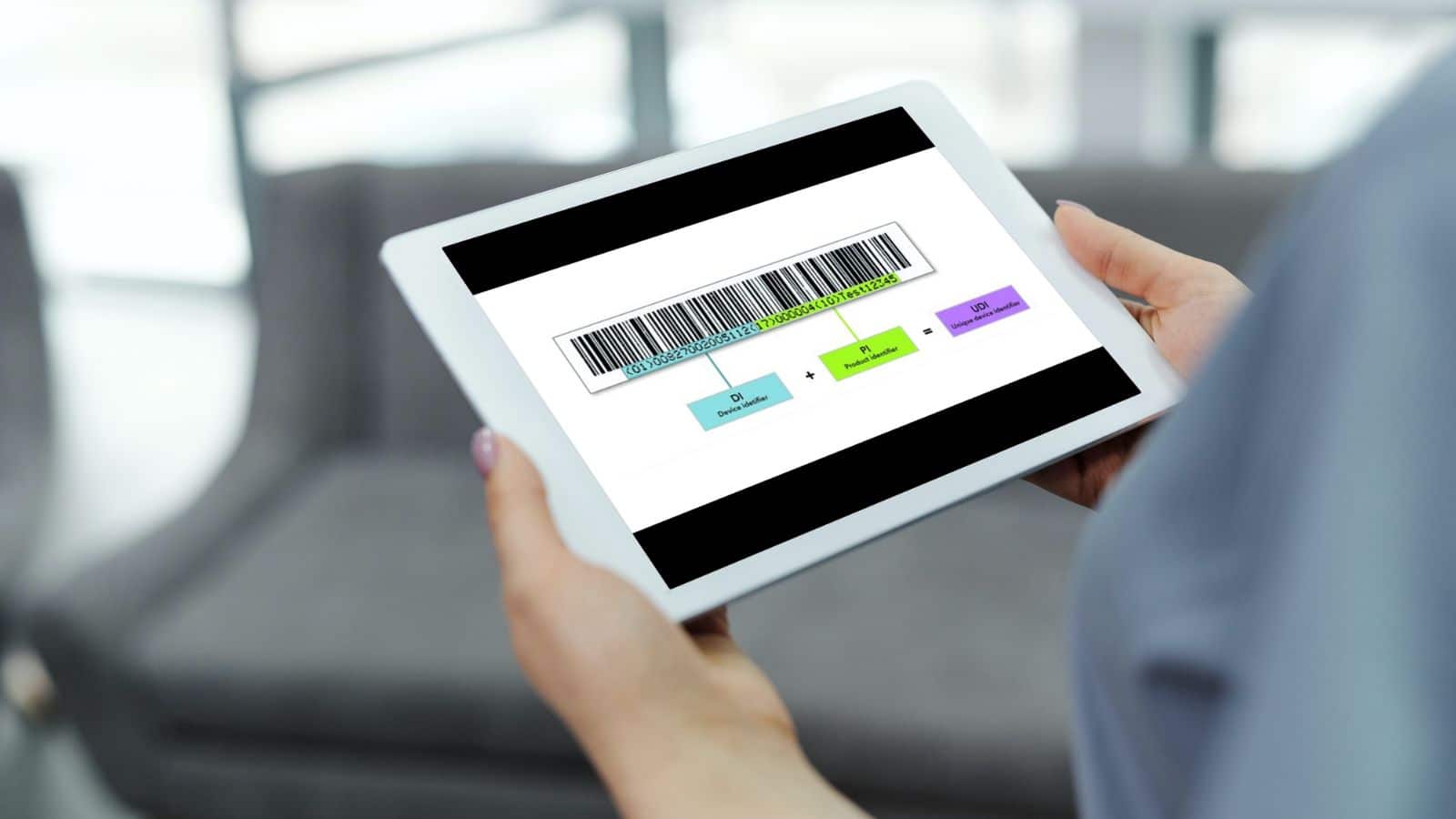
We recently published a blog post about Unique Device Identifiers (UDI). While this blog post briefl...

 No Items Found! Please Try Some Different Keywords
No Items Found! Please Try Some Different Keywords

We recently published a blog post about Unique Device Identifiers (UDI). While this blog post briefl...
Are you aware that the introduction of MDR may have affected your CE-marked medical software (MDSW)?...
Nowadays, digital healthcare is taking an increasingly important place in the medical world through ...
What is a medical device? This crucial question often arises when bringing medical devices to market...