- verify the consistency of existing process parameters,
- the accuracy of current specifications of both raw materials and finished products,
- and to uncover specific information to identify product and process improvements.
Why is it important to conduct an Annual Product Quality Review (APQR) in Pharma?
Annual Product Quality Review is not only a legal requirement but also allows the company to better understand the processes and take improvement actions to ensure product quality.
In pharma, APQR / PQR determines the need for changes to the manufacturing process, in-process controls, and specifications. This involves identifying improvements to the product and manufacturing process based on trend analysis and risk assessment.
The objectives of the PQR include:
-
Verification of product performance.
-
Consistency of the manufacturing process.
-
Determination of the need for revalidation of manufacturing processes.
What are the benefits of a Product Quality Review (PQR)?
The APQR / PQR helps identify and prioritize improvement opportunities, communicate improvement recommendations from product reviews to management and meet regulatory requirements, as well as assess the validated status of processes. Some benefits are listed below:
- Ensures Product Quality
- Facilitates the adjustment of process, raw material and excipient specifications
- Process improvement
- Helps identify areas of improvement to adjust processing and control procedures
- Reduction of quality defects
- Better understanding of production processes and identification of tendencies
- Identification of potential risks due to variations in processes and materials
- Compliance with regulatory authority requirements
- Generates a consistent, efficient and effective way of working
- Encourages a culture of prevention rather than reaction
- Better product knowledge
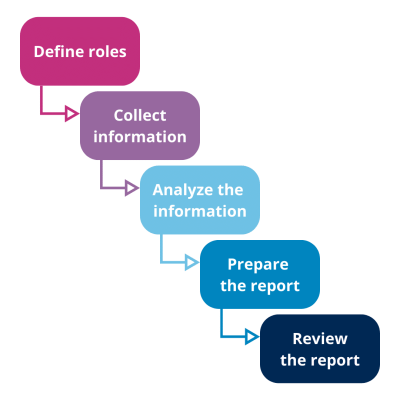
General stages of the Product Quality Review Process in Pharma
Elements to consider for the Product Quality Review
Common details for the APQR / PQR
- Product Identification
- Documentation; formula, specifications
- Batches manufactured (approved, rejected)
- Raw and packaging materials
- Deviations, out-of-specification results, rework, rejects, recalls, investigation, resolution, and associated action plans (CAPAs)
- Complaints, returns and recalls
- Supplier Qualification
- Critical quality attributes and critical process parameters
- Qualification status of areas, equipment and systems and their impact on production process validation and analytical methods
- Retention samples
- Results of stability studies
- Outsourced activities, technical agreements and contracts with third parties
- Change controls / Changes in registration conditions
- Post-marketing commitments
- Adverse reactions
- Resulting actions from previous APQR
- Resulting actions arising from APQR conclusions
Elements to consider when reporting
- Objective / Scope
- Review of processed batches
- Change Control Review (consider label and gear / artwork changes)
- Review of analytical data
- Stability data review
- Validation and qualification review
- Review of nonconformities/deviations
- Review of rejected lots
- Review of reprocessed batches
- Claims review
- Recall review
- Counter-sample review
- Quality agreements
- Review of previous APRs
- Conclusions and recommendations
- References
- Approval
Key challenges in conducting a Product Quality Review (APQR / PQR) in Pharma
Weekly batch data reports
Every week, you need to get reports of batch data, including production, packaging, and other reports. Collecting raw data is always an effort; there must be someone responsible for getting and compiling all the information.
Often, this is someone from the quality department, someone who focuses on these tasks, and there must also be planning and management of tasks and responsibilities so that data, reports, or other related information is delivered according to plan.
This means you will need to assign one or two people to manage these tasks.
Data retrieval for evaluation purposes
Another challenge in conducting a PQR is data retrieval for evaluation purposes.
If your company has an automated system for obtaining qualified data, its database can be used. However, if you do not have such a system, reviewing individual batches on paper to assess process parameters, process controls, statistical analysis, and batch performance can be cumbersome and time-consuming.
In both of the above cases, the raw data used for analysis must be accurate for effective analysis and evaluation. If process deviations are found during the assessment, additional information may need to be collected to justify such findings.
Data availability to the PQR manager
Data should be available to the PQR manager at all times and should be verified by a second person if collected manually.
Validated spreadsheets
If spreadsheets are used, they should be validated before use.
Considerations about the Annual Product Quality Review (APQR / PQR) in Pharma
Annual systematic quality review
An annual systematic quality review of each product should be conducted. The QA department must ensure implementation of the PQR planning and designate the person responsible for its implementation and dissemination.
- The objectives of the PQR are verification of product performance, consistency of the manufacturing process and determination of the need for revalidation of manufacturing processes.
- PQR determines the need for manufacturing process changes, in-process controls and specifications. This includes identification of improvements to the product and manufacturing process based on trend analysis and risk assessment.
- Grouping of products is not allowed regardless of whether similar processes and equipment are used in their manufacture.
- A PQR report must be available for imported drugs, which must contain the information generated by the manufacturer and must be supplemented by the information generated by the processes carried out in the national territory.
PQR implementation procedure
There should be a procedure for implementing the PQR that includes the objectives for determining and justifying the areas selected for review and the potential scope of the review.
Formatted summary
The results of the PQR should be summarized in a specified format.
Why consider conducting your Annual Product Quality Review (APQR / PQR) with QbD Group?
-
-
No need to assign your own staff to these activities. Remember: depending on your processes, you may need to assign one, two or even three people to perform Product Quality Review activities!
-
Say goodbye to software licensing for statistical review. You don’t need to pay for licenses for all people involved or buy software for data analysis and statistical management.
-
Provide us with the information from Production, Packaging, QA and QC, and we provide the PQR reporting, whether weekly, monthly or annually. Rest assured that we will include improvement points and trend graphs in the report.
-
The APQR reports will be ready in time. Forget about PQR scheduling, follow-up with affected areas and long wait times. We will do it for you!
-
You will have the information you need at any time, in a database available 24/7.
-







.png?width=109&height=108&name=Pharma%20(2).png)
.png?width=111&height=108&name=Medical%20Devices%20(2).png)
.png?width=84&height=107&name=IVD%20(2).png)










.png)

.jpg)
